Tsarin carbon
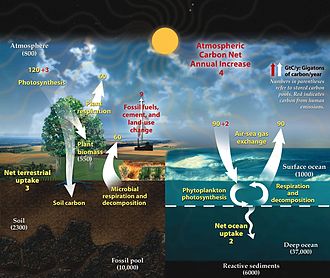
Tsarin carbon shine wannan bangare na sake zagayowar biogeochemical wanda ake musayar carbon tsakanin biosphere, pedosphere, geosphere, hydrosphere, da yanayi na Duniya. Sauran manyan sake zagayowar biogeochemical sun haɗa da sake zagayolar nitrogen da sake zagaye na ruwa. Carbon shine babban bangare na mahadi na halitta da kuma babban bangare ne na ma'adanai da yawa kamar dutse. Tsarin carbon ya ƙunshi jerin abubuwan da suka faru waɗanda keda mahimmanci don sa Duniya ta iya kiyaye rayuwa. Ya bayyana motsi na carbon yayin da aka sake amfani dashi kuma aka sake amfani dashi a duk faɗin biosphere, da kuma matakai na dogon lokaci na ƙwaƙwalwar carbon (ajiyewa) zuwa da saki daga sinks na carbon.
Don bayyana yanayin sake zagayowar carbon, ana iya yin bambanci tsakanin saurin saurin sautin carbon. Ana kuma kiran sake zagayowar carbon mai sauri a matsayin sake zagayolar carbon na halitta. Saurin zagaye na carbon na iya kammalawa cikin shekaru, motsa abubuwa daga yanayi zuwa biosphere, sannan komawa cikin yanayi. Saurin ko sake zagayowar ƙasa (wanda ake kira zurfin sake zagayolar carbon) na iya ɗaukar miliyoyin shekaru don kammalawa, abubuwa masu motsi ta hanyar ɓawon burodi na Duniya tsakanin duwatsu, ƙasa, teku da yanayi.[2]
Mutane sun dame sake zagayowar carbon na ƙarni da yawa. Sunyi hakan ta hanyar canza amfani da ƙasa da kuma hakar ma'adinai da ƙone carbon daga tsoffin kayan halitta (kwal, man fetur da gas). [1] Carbon dioxide a cikin yanayi yakaru kusan 52% a kan matakan da suka gabata zuwa 2020, wanda ya haifar da dumamar duniya.[3] Karin carbon dioxide ya kuma haifar da raguwa a cikin darajar pH na teku kuma yana canza sunadarai na ruwa.[4] Carbon dioxide yana da mahimmanci ga photosynthesis.
Babban ɗakuna
[gyara sashe | gyara masomin]An nuna motsi na carbon na ƙasa a cikin sake zagayowar ruwa a cikin zane a dama kuma an bayyana shi a ƙasa:
- Yanayi
- Yanayin halittu na duniya
- Tekun, gami da narkewar carbon inorganic da rayayyun halittu masu rai da wadanda ba rayayyun ruwa ba
- Rashin ruwa, gami da man fetur, tsarin ruwa mai laushi, da kayan halitta marasa rai.
- Cikin duniya (mantle da ɓawon burodi). Wadannan shagunan carbon suna hulɗa tare da sauran abubuwan ta hanyar hanyoyin ilimin ƙasa.
Canjin carbon tsakanin tafkuna yana faruwa ne sakamakon tsarin sunadarai, na jiki, na ƙasa, da na halitta. Tekun ya ƙunshi mafi girman tafkin carbon mai aiki kusa da farfajiyar Duniya.[5]Hanyar halitta ta carbon tsakanin yanayi, teku, yanayin halittu na ƙasa, da kuma turɓaya suna da daidaituwa sosai; don haka matakan carbon zasu kasance kusan kwanciyar hankali batare da tasirin ɗan adam ba.[6]
Yanayi
[gyara sashe | gyara masomin]Carbon a cikin yanayin duniya yana cikin manyan siffofi guda biyu: carbon dioxide da methane. Dukkanin wadannan iskar gas suna shanyewa kuma suna riƙe da zafi a cikin yanayi kuma suna da wani bangare na alhakin tasirin greenhouse.[5] Methane yana samar da babban tasirin greenhouse a kowane girma idan aka kwatanta da carbon dioxide, amma yana cikin ƙananan maida hankali kuma yana da ɗan gajeren lokaci fiye da carbon dioxide. Don haka, carbon dioxide yana ba da gudummawa ga tasirin greenhouse na duniya fiye da methane.[8]
Ana cire carbon dioxide daga yanayi da farko ta hanyar photosynthesis kuma ya shiga cikin yanayin ƙasa da na teku. Har ila yau, carbon dioxide yana narkewa kai tsaye daga yanayi zuwa cikin ruwa (teku, tabkuna, da dai sauransu), kazalika da narkewa cikin hazo yayin da ruwan sama yafadi ta cikin yanayi. Lokacin da aka narke a cikin ruwa, carbon dioxide yana amsawa tare da kwayoyin ruwa kuma yana samar da carbonic acid, wanda ke bada gudummawa ga acidity na teku. Sa'an nan kuma duwatsu zasu iya shawo kansa ta hanyar yanayi. Hakanan yana iya yin acid a wasu wurare da ya taɓa ko kuma a wanke shi cikin teku.[9]

Ayyukan ɗan adam a cikin ƙarni biyu da suka gabata sun ƙara adadin carbon a cikin yanayi da kusan kashi 50% tun daga shekara ta 2020, galibi a cikin nau'in carbon dioxide, duka ta hanyar canza ikon yanayin halittu don cire carbon dioxide daga yanayi da kuma fitar dashi kai tsaye, misali, ta hanyar ƙone burbushin burbushin halittu da ƙera kankare.[3][5] A nan gaba mai nisa (shekaru biliyan 2 zuwa 3), yawan da ake shan carbon dioxide a cikin ƙasa ta hanyar sake zagayowar carbonate-silicate zai iya ƙaruwa saboda canje-canjen da ake tsammani a cikin rana yayin da yake tsufa. Ƙarin hasken rana da ake tsammani zai iya hanzarta yawan yanayin ƙasa.[10] Wannan zai haifar da mafi yawan carbon dioxide a cikin sararin samaniya ya shiga cikin ɓawon burodi na Duniya a matsayin carbonate.[11][12] Da zarar maida hankali ga carbon dioxide a cikin yanayi yafaɗi ƙasa da kusan kashi 50 a kowace miliyan (haƙuri ya bambanta tsakanin jinsuna), C<sub id="mwjg">3</sub> photosynthesis bazai yiwu ba.[13] Anyi hasashen wannan zai faru shekaru miliyan 600 daga yanzu, kodayake samfuran sun bambanta.[14]
Da zarar teku a Duniya ta bushe a cikin kimanin shekaru biliyan 1.1 daga yanzu, [10] farantin tectonics zai iya tsayawa saboda rashin ruwa don shafa su. Rashin tsaunuka masu fashewa dake fitar da carbon dioxide zai haifar da sake zagayowar carbon ya ƙare tsakanin shekaru biliyan 1 zuwa biliyan 2 a nan gaba.[15]
Yanayin halittu na duniya
[gyara sashe | gyara masomin]
Tsarin halittu na duniya ya haɗa da carbon na halitta a cikin dukkan kwayoyin halitta masu rai, masu rai da matattu, da kuma carbon da aka adana a cikin ƙasa. Kimanin 500 gigatons na carbon ana adana su a sama da ƙasa a cikin tsire-tsire da sauran kwayoyin halitta masu rai, yayin da ƙasa ke riƙe da kusan 1,500 gigatons of carbon. [16] Yawancin carbon a cikin yanayin halittu na ƙasa shine carbon na halitta, yayin da kusan kashi ɗaya bisa uku na carbon na ƙasa ana adana shi a cikin siffofin inorganic, kamar calcium carbonate. [17][18] Organic carbon babban bangare ne na dukkan kwayoyin dake rayuwa a duniya. Autotrophs suna cire shi daga iska a cikin nau'in carbon dioxide, suna canza shi zuwa carbon na halitta, yayin da heterotrophs ke karɓar carbon ta hanyar cinye wasu kwayoyin.
Saboda karɓar carbon a cikin yanayin halittu na ƙasa ya dogara da abubuwan da ke tattare da halittu, yana bin tsarin rana da na yanayi. A cikin ma'aunin CO2, wannan fasalin ya bayyana a cikin keeling curve. Yana da karfi a arewacin arewacin arewa saboda wannan arewacin yana da ƙasa da yawa fiye da kudancin kudancin kuma ta haka ne ƙarin wuri ga yanayin halittu don shawo kan kuma fitar da carbon.

Carbon leaves the terrestrial biosphere in several ways and on different time scales. The combustion or respiration of organic carbon releases it rapidly into the atmosphere. It can also be exported into the ocean through rivers or remain sequestered in soils in the form of inert carbon.[19] Carbon stored in soil can remain there for up to thousands of years before being washed into rivers by erosion or released into the atmosphere through soil respiration. Between 1989 and 2008 soil respiration increased by about 0.1% per year.[20] In 2008, the global total of CO2 released by soil respiration was roughly 98 billion tonnes[ana buƙatar hujja], about 3 times more carbon than humans are now putting into the atmosphere each year by burning fossil fuel (this does not represent a net transfer of carbon from soil to atmosphere, as the respiration is largely offset by inputs to soil carbon).[ana buƙatar hujja][<span title="This claim needs references to reliable sources. (April 2024)">citation needed</span>] There are a few plausible explanations for this trend, but the most likely explanation is that increasing temperatures have increased rates of decomposition of soil organic matter, which has increased the flow of CO2. The length of carbon sequestering in soil is dependent on local climatic conditions and thus changes in the course of climate change.[21]
| Ruwa | Adadin (gigatons) |
|---|---|
| Yanayi | 720 |
| Tekun (cikakken) | 38,400 |
| Cikakken inorganic | 37,400 |
| Cikakken kwayoyin halitta | 1,000 |
| Layer na sama | 670 |
| Layer mai zurfi | 36,730 |
| Lithosphere | |
| Carbonates na sedimentary | > 60,000,000 |
| Kerogens | 15,000,000 |
| Biosphere na duniya (cikakken) | 2,000 |
| Rayayyun halittu | 600 – 1,000 |
| Matattu biomass | 1,200 |
| Yanayin halittu na ruwa | 1 – 2 |
| Abubuwan da ke cikin burbushin halittu (cikakken) | 4,130 |
| Karfe | 3,510 |
| Mai | 230 |
| Gas | 140 |
| Sauran (peat) | 250 |
Tekun
[gyara sashe | gyara masomin]Za'a iya raba teku a cikin wani nau'i na sama wanda ruwa ke yin hulɗa akai-akai (kowace zuwa shekara-shekara) tare da yanayi, da kuma zurfin zurfin zurfi na mita ɗari ko ƙasa da haka, wanda lokacin tsakanin lambobin sadarwa na gaba zai iya zama ƙarni. Ana musayar carbon inorganic (DIC) da aka narke a cikin farfajiyar da sauri tare da yanayi, yana riƙe da daidaituwa. Wani bangare saboda yawan DIC ya kai kusan 15% mafi girma amma galibi saboda girmansa, teku mai zurfi ya ƙunshi carbon da yawa - shine mafi girman tafkin carbon mai aiki a duniya, wanda ke dauke da sau 50 fiye da yanayi [5] amma lokacin da zafin zai kai ga daidaito tare da yanayi shine daruruwan shekaru: musayar carbon tsakanin yadudduka biyu, wanda ke motsawa ta hanyar zagayawar thermohaline, yana da jinkiri.
Carbon ya shiga cikin teku galibi ta hanyar rushewar carbon dioxide na yanayi, karamin ɓangaren da aka canza zuwa carbonate. Hakanan yana iya shiga cikin teku ta hanyar koguna a matsayin carbon mai narkewa. Kwayoyin halitta suna canza shi zuwa carbon na kwayoyin halitta ta hanyar photosynthesis kuma ana iya musayar shi a ko'ina cikin jerin abinci ko kuma a kwashe shi cikin zurfin teku, mafi wadataccen carbon a matsayin matattu mai laushi ko a cikin harsashi kamar calcium carbonate. Yana zagayawa a cikin wannan Layer na dogon lokaci kafin a ajiye shi a matsayin laka ko, a ƙarshe, ya koma cikin ruwa ta hanyar zagayawar thermohaline.
Tekuna na asali ne (tare da Darajar pH na yanzu na 8.1 zuwa 8.2). Karin CO2 na yanayi yana canza pH na teku zuwa tsaka-tsaki a cikin tsari da ake kira acidisation na teku. Rashin CO2 na teku yana daya daga cikin mahimman nau'ikan carbon. Yawan da aka tsara na raguwar pH na iya rage saurin hazo na calcium carbonates, don haka rage ikon teku don sha CO2. [22][23]
Geosphere
[gyara sashe | gyara masomin]
An nuna motsi na carbon na ƙasa a cikin sake zagayowar ruwa a cikin zane a dama kuma an bayyana shi a ƙasa:
Yawancin carbon na Duniya ana adana su a cikin lithosphere na Duniya.[5] Yawancin carbon da aka adana a cikin mantle na Duniya an adana shi a can lokacin da aka kafa Duniya. Wasu daga cikinsu an ajiye su a cikin nau'in carbon na kwayoyin halitta daga biosphere.[24] Daga cikin carbon da aka adana a cikin geosphere, kusan 80% shine dutse mai laushi da abubuwan da aka samo asali, wanda ya samo asali ne daga sedimentation na calcium carbonate da aka adada a cikin kwarangwal na kwayoyin ruwa. Sauran kashi 20% ana adana su azaman kerogens da aka kafa ta hanyar sedimentation da binne kwayoyin ƙasa a ƙarƙashin zafi da matsin lamba. Kwayar carbon da aka adana a cikin geosphere na iya kasancewa a can na miliyoyin shekaru.[25]
Carbon na iya barin geosphere ta hanyoyi da yawa. Ana fitar da carbon dioxide a lokacin metamorphism na duwatsun carbonate lokacin da aka saukar dasu cikin mantle na duniya. Ana iya sakin wannan carbon dioxide a cikin yanayi da teku ta hanyar tsaunuka masu fitattun wuta da hotspots. Hakanan mutane na iya cire shi ta hanyar cirewa kai tsaye na kerogens a cikin nau'in man fetur. Bayan cirewa, ana ƙone burbushin burbushin don fitar da makamashi da fitar da carbon da suke adanawa cikin yanayi.
Nau'o'in ƙarfin
[gyara sashe | gyara masomin]
The fast carbon cycle operates through the biosphere, see diagram at start of article ↑
Akwai saurin sake zagayowar carbon. Tsarin sauri yana aiki a cikin biosphere kuma jinkirin sake zagayowar yana aiki a kan duwatsu. Tsarin sauri ko na halitta na iya cikawa cikin shekaru, yana motsa carbon daga yanayi zuwa biosphere, sannan ya koma cikin yanayi. Saurin ko sake zagayowar ƙasa na iya fadada zurfi cikin mantle kuma yana iya ɗaukar miliyoyin shekaru don kammalawa, yana motsa carbon ta cikin ɓawon duniya tsakanin duwatsu, ƙasa, teku da yanayi.[2]
The fast carbon cycle involves relatively short-term biogeochemical processes between the environment and living organisms in the biosphere (see diagram at start of article). It includes movements of carbon between the atmosphere and terrestrial and marine ecosystems, as well as soils and seafloor sediments. The fast cycle includes annual cycles involving photosynthesis and decadal cycles involving vegetative growth and decomposition. The reactions of the fast carbon cycle to human activities will determine many of the more immediate impacts of climate change.[26][27][28]
Saurin (ko zurfi) sake zagayowar carbon ya haɗa da matsakaici zuwa matakai na geochemical na dogon lokaci na cikin sake zagayolar dutse (duba zane a dama). Canjin tsakanin teku da yanayi na iya ɗaukar ƙarni, kuma yanayin duwatsu na iya ɗaukar miliyoyin shekaru. Carbon a cikin teku yana sauka zuwa ƙarƙashin teku inda zai iya samar da dutse mai laushi kuma a subducted cikin mantle na Duniya. Hanyoyin gine-ginen dutse suna haifar da dawowar wannan carbon na ƙasa zuwa farfajiyar Duniya. A can ana samun iska a kan duwatsu kuma ana mayar da carbon zuwa yanayi ta hanyar degassing da kuma teku ta koguna. Sauran carbon na geologic ya koma cikin teku ta hanyar fitar da hydrothermal na ions na calcium. A cikin shekara guda tsakanin tan miliyan 10 zuwa 100 na carbon yana motsawa a kusa da wannan jinkirin sake zagayowar. Wannan ya haɗa da tsaunuka masu fashewa da ke dawo da carbon kai tsaye zuwa yanayi a cikin nau'in carbon dioxide. Koyaya, wannan ƙasa da kashi ɗaya cikin ɗari ne na carbon dioxide da aka sanya cikin yanayi ta hanyar ƙone man fetur.[2][29]
Ƙananan matakai a cikin saurin sake zagayowar carbon
[gyara sashe | gyara masomin]Carbon na ƙasa a cikin sake zagayowar ruwa
[gyara sashe | gyara masomin]
An nuna motsi na carbon na ƙasa a cikin sake zagayowar ruwa a cikin zane a dama kuma an bayyana shi a ƙasa:
- Atmospheric particles act as cloud condensation nuclei, promoting cloud formation.[31][32]
- Raindrops absorb organic and inorganic carbon through particle scavenging and adsorption of organic vapors while falling toward Earth.[33][34]
- Burning and volcanic eruptions produce highly condensed polycyclic aromatic molecules (i.e. black carbon) that is returned to the atmosphere along with greenhouse gases such as CO2.[35][36]
- Terrestrial plants fix atmospheric CO2 through photosynthesis, returning a fraction back to the atmosphere through respiration.[37] Lignin and celluloses represent as much as 80% of the organic carbon in forests and 60% in pastures.[38][39]
- Litterfall and root organic carbon mix with sedimentary material to form organic soils where plant-derived and petrogenic organic carbon is both stored and transformed by microbial and fungal activity.[40][41][42]
- Water absorbs plant and settled aerosol-derived dissolved organic carbon (DOC) and dissolved inorganic carbon (DIC) as it passes over forest canopies (i.e. throughfall) and along plant trunks/stems (i.e. stemflow).[43] Biogeochemical transformations take place as water soaks into soil solution and groundwater reservoirs[44][45] and overland flow occurs when soils are completely saturated, or rainfall occurs more rapidly than saturation into soils.[46]
- Organic carbon derived from the terrestrial biosphere and in situ primary production is decomposed by microbial communities in rivers and streams along with physical decomposition (i.e. photo-oxidation), resulting in a flux of CO2 from rivers to the atmosphere that are the same order of magnitude as the amount of carbon sequestered annually by the terrestrial biosphere.[47][48][49] Terrestrially-derived macromolecules such as ligninSamfuri:Hsp[50] and black carbonSamfuri:Hsp[51] are decomposed into smaller components and monomers, ultimately being converted to CO2, metabolic intermediates, or biomass.
- Lakes, reservoirs, and floodplains typically store large amounts of organic carbon and sediments, but also experience net heterotrophy in the water column, resulting in a net flux of CO2 to the atmosphere that is roughly one order of magnitude less than rivers.[52][49] Methane production is also typically high in the anoxic sediments of floodplains, lakes, and reservoirs.[53]
- Primary production is typically enhanced in river plumes due to the export of fluvial nutrients.[54][55] Nevertheless, estuarine waters are a source of CO2 to the atmosphere, globally.[56]
- Coastal marshes both store and export blue carbon.[57][58] Marshes and wetlands are suggested to have an equivalent flux of CO2 to the atmosphere as rivers, globally.[59]
- Continental shelves and the open ocean typically absorb CO2 from the atmosphere.[56]
- The marine biological pump sequesters a small but significant fraction of the absorbed CO2 as organic carbon in marine sediments (see below).[60]
Ruwa na ƙasa zuwa teku
[gyara sashe | gyara masomin]
Tsarin halittu na ƙasa da na ruwa galibi ana haɗa su ta hanyar jigilar kogin, wanda ke aiki a matsayin babban tashar da abubuwan da aka samo daga ƙasa suka shiga cikin tsarin teku. Canjin kayan aiki da makamashi tsakanin Yanayin halittu na ƙasa da lithosphere da kuma tsarin carbon na kwayoyin halitta da hanyoyin oxidation tare suna tsara tsarin halittu na carbon da dioxygen (O2). [61]
Sufuri na kogi, kasancewa babbar hanyar haɗi ta waɗannan tafkuna, zatayi aiki don jigilar yawan amfanin gona na farko (da farko a cikin nau'in narkewar carbon (DOC) da carbon carbon mai ƙwayoyin cuta (POC)) daga ƙasa zuwa tsarin teku.[62] A lokacin sufuri, wani ɓangare na DOC zai dawo cikin sauri zuwa yanayi ta hanyar halayen redox, wanda ke haifar da "degasing carbon" yafaru tsakanin yadudduka na ajiyar ƙasa.[63][64] Sauran DOC da narkewar carbon inorganic (DIC) ana fitar dasu zuwa teku.[65][66][67] A cikin 2015, an kimanta yaduwar fitar da carbon na inorganic da kwayoyin halitta daga koguna na duniya a matsayin 0.50-0.70 Pg C y-1 da 0.15-0.35 Pg C da-1 bi da bi.[66] A gefe guda, POC na iya kasancewa a binne shi a cikin turɓaya a tsawon lokaci, kuma an kiyasta ƙarar duniya ta shekara-shekara zuwa teku a 0.20 (+0.13,-0.07) Gg C y−1 . [68][61]
Fumbar halittu a cikin teku
[gyara sashe | gyara masomin]
Fump din halittu na teku shine kwacewar carbon ta teku daga yanayi da ƙasa zuwa zurfin teku da kuma zurfin teku.[69] Fump din halittu ba sakamakon tsari ɗaya ba ne, amma jimlar matakai da yawa kowannensu na iya rinjayar famfo na halittu. Fuskar tana canja wurin kimanin tan biliyan 11 na carbon a kowace shekara zuwa cikin teku. Tekun ba tare da famfo na halitta bazai haifar da matakan CO2 na yanayi game da 400 ppm sama da na yau.[70][71][72]
Yawancin carbon da aka haɗa a cikin kwayoyin halitta da kwayoyin halitta an kafa sune a saman teku inda zai iya fara nutsewa zuwa ƙarƙashin teku. Ruwa mai zurfi yana samun mafi yawan abubuwan gina jiki daga ginshiƙan ruwa mafi girma lokacin da suka nutse a cikin nau'in dusar ƙanƙara. Wannan ya kunshi dabbobi da ƙwayoyin cuta da matattu ko masu mutuwa, ƙwayoyin ƙwayoyin, yashi da sauran kayan inorganic.[73]
Fumbar halittu tana da alhakin canza narkewar carbon inorganic (DIC) zuwa kwayoyin halitta da kuma jefa shi a cikin ƙwayoyin cuta ko narkewar tsari a cikin teku mai zurfi. Inorganic nutrients da carbon dioxide suna gyara a lokacin photosynthesis ta hanyar phytoplankton, wanda duka biyu suna fitar da kwayoyin halitta (DOM) kuma zooplankton mai cin ganyayyaki ke cinye su. Babban zooplankton - kamar copepods, ƙwayoyin ƙwayoyin cuta - wanda za'a iya sake dawowa, kuma ya nutse ko ya tattara tare da wasu kayan ƙwayoyin halitta a cikin manyan abubuwa masu saurin nutsewa. DOM yana cinyewa da ƙwayoyin cuta kuma yana numfashi; sauran DOM mai ƙin yarda suna advected kuma suna gaurayewa cikin zurfin teku. DOM da abubuwan da aka fitar da su cikin ruwa mai zurfi ana cinye su kuma ana numfasawa, don haka dawo da carbon na halitta a cikin babban tafkin teku mai zurfi na DIC.[74]
Kwayar phytoplankton guda ɗaya tana da ƙimar nutsewa kusan mita ɗaya a kowace rana. Idan aka bada cewa matsakaicin zurfin teku kusan kilomita huɗu ne, zai iya ɗaukar sama da shekaru goma don waɗannan sel su isa ƙarƙashin teku. Koyaya, ta hanyar matakai irin su coagulation da fitarwa a cikin ƙwayoyin ƙwayoyin cuta, waɗannan ƙwayoyin suna samar da tarawa. Wadannan tarin suna da ƙididdigar nutsewa dayawa fiye da ƙwayoyin halitta kuma sun kammala tafiyarsu zuwa zurfi a cikin kwanaki.
Kimanin kashi 1% na barbashi dake barin teku sun kai ga zurfin teku kuma ana cinye su, numfashi, ko binne su a cikin turɓaya. Sakamakon waɗannan matakai shine cire carbon a cikin nau'in kwayoyin halitta daga farfajiya kuma mayar dashi zuwa DIC a zurfin zurfi, kiyaye yanayin teku na DIC. Yankin thermohaline ya dawo da zurfin teku DIC zuwa yanayi a kan lokutan dubban shekaru. Ana iya saukar da carbon da aka binne a cikin turɓaya a cikin mantle na duniya kuma a adana shi na miliyoyin shekaru a matsayin wani ɓangare na jinkirin sake zagayowar carbon (duba sashi na gaba). [74]
Ƙananan matakai a cikin jinkirin sake zagayowar carbon
[gyara sashe | gyara masomin]
Saurin ko zurfin cycling yana da mahimmanci, kodayake ba'a fahimta sosai kamar yadda motsi na carbon mai sauri ta hanyar yanayi, yanayin ƙasa, teku, da geosphere ba.[75] Tsarin carbon mai zurfi yana da alaƙa da motsi na carbon a farfajiyar duniya da yanayi. Idan ba'a wanzu wannan tsari ba, carbon zai kasance a cikin yanayi, inda zai tara zuwa matakai masu girma a tsawon lokaci. Sabili da haka, ta hanyar bada izinin carbon ya koma Duniya, zurfin sake zagayowar carbon yana taka muhimmiyar rawa wajen kiyaye yanayin duniya da ake buƙata don rayuwa ta wanzu.
Bugu da ƙari, wannan tsari yanads mahimmanci kawai saboda yawan carbon da yake ɗauka a cikin duniyar. A zahiri, nazarin abun dake cikin basaltic Magma da auna iskar carbon dioxide daga dutsen wuta ya nuna cewa adadin carbon a cikin mantle yafi na duniya girma da kashi dubu daya.[76] Bincike da lura da matakai masu zurfin carbon na duniya a bayyane yake yana da matukar wahala, yayin da ƙananan mantle da core suka shimfiɗa daga 660 zuwa 2,891 km da 2,891 zuwa 6,371 km zurfi a cikin Duniya bi da bi. Dangane da haka, basan abubuwa da yawa game da rawar da carbon ke takawa a cikin zurfin Duniya ba. Duk da haka, shaidu da yawa - da yawa daga cikinsu sun fito ne daga gwajin dakin gwaje-gwaje na yanayin duniya mai zurfi - sun nuna hanyoyin da za su iya motsawa a cikin ƙananan mantle, da kuma siffofin da carbon ke ɗauka a matsanancin yanayin zafi da matsin lamba na wannan Layer. Bugu da ƙari, dabaru kamar seismology sun haifar da ƙarin fahimtar yiwuwar kasancewar carbon a cikin ainihin Duniya.
Carbon a cikin ƙananan mantle
[gyara sashe | gyara masomin]
Carbon yafi shiga cikin mantle a cikin nau'in carbonate-mai wadataccen sediments a kan tectonic faranti na teku, wanda ke jan carbon cikin mantle bayan da ya sha wahala. Ba a san abubuwa da yawa game da yaduwar carbon a cikin mantle ba, musamman a cikin zurfin Duniya, amma bincike da yawa sun yi ƙoƙari su kara fahimtarmu game da motsi da siffofin kashi a cikin yankin. Misali, binciken da akayi a shekara ta 2011 ya nuna cewa zagaye na carbon yakai har zuwa ƙananan mantle. Binciken yayi nazarin lu'u-lu'u masu zurfi a wani shafin a Juina, Brazil, yana ƙayyade cewa yawancin abubuwan da ke cikin wasu lu'u'u-ulu'u sun dace da sakamakon da ake tsammani na narkewar basalt da crystallisation a ƙarƙashin ƙananan yanayin zafi da matsin lamba. Don haka, binciken binciken ya nuna cewa ɓangarorin basaltic oceanic lithosphere suna aiki a matsayin ƙa'idar hanyar sufuri don carbon zuwa zurfin ciki na Duniya. Wadannan carbonates da aka rage zasu iya hulɗa tare da ƙananan silicates, a ƙarshe suna samar da lu'u-lu'u masu zurfi kamar wanda aka samu.[78]
Koyaya, carbonates da ke saukowa zuwa ƙananan mantle suna haɗuwa da wasu abubuwan da suka faru banda samar da lu'u-lu'u. A cikin shekara ta 2011, an sanya carbonates a cikin yanayin da yayi kama da na zurfin kilomita 1800 a cikin Duniya, da kyau a cikin ƙananan mantle. Yin haka ya haifar da tsarin Magnesite, siderite, da nau'ikan nau'ikan graphite.[79] Sauran gwaje-gwaje - kazalika da abubuwan lura na petrologic - suna tallafawa wannan da'awar, suna nuna cewa magnesite shine ainihin matakin carbonate mafi tsayayya a mafi yawan ɓangaren mantle. Wannan galibi sakamakon yawan zafin jiki ne.[80] Sakamakon haka, masana kimiyya sun kammala cewa carbonates suna fuskantar raguwa yayin da suke sauka cikin mantle kafin a daidaita su a zurfi ta hanyar yanayin oxygen fugacity. Magnesium, baƙin ƙarfe, da sauran mahadi na ƙarfe suna aiki azaman buffers a duk lokacin da aka aiwatar.[81] Kasancewar ragewa, siffofin carbon kamar graphite zai nuna cewa mahaɗan carbon suna raguwa yayin da suke sauka cikin mantle.

Binciken Kasuwancin Majalisar Dinkin Duniya na 2009-2010 ya ba da rahoton cewa kamfanonin da suka samar ko suka yi ciniki a cikin samfuran gandun daji masu inganci galibi suna da fa'idar kasuwa a lokacin koma bayan tattalin arziki na 2008-2009 saboda, a cikin kasuwar masu siye, masu siye na iya zama mafi zaɓaɓɓu wajen zaɓar hanyoyin samar da su. Rahoton ya ambaci direbobi huɗu da ake buƙata don takaddun shaida: yana canza kwanciyar hankali na mahaɗan carbonate a zurfi daban-daban a cikin Duniya. Don kwatanta, ƙididdigar dakin gwaje-gwaje da ƙididdigaren ka'idar aiki mai yawa sun nuna cewa carbonates da aka daidaita da tetrahedrally sun fi kwanciyar hankali a zurfin da ke kusa da iyakar core-mantle.[82][83] Wani binciken da akayi a shekarar 2 ya nuna cewa matsin lamba na ƙananan mantle yana haifar da haɗin carbon zuwa sauyawa daga sp2 zuwa sp3 Hybridized orbitals, wanda ke haifar da haɗin haɗin carbon tetrahedrally zuwa oxygen.[84] Ƙungiyoyin CO3 trigonal ba zasu iya samar da cibiyoyin sadarwa masu polymerisable ba, yayin da CO4 na tetrahedral zai iya, yana nuna karuwar lambar daidaitawa carbon, sabili da haka canje-canje masu yawa a cikin kaddarorin carbonate a cikin ƙananan mantle. A matsayin misali, binciken farko na ka'idoji ya nuna cewa matsin lamba yana haifar da ƙarancin narkewar carbonate don ƙaruwa; ƙarancin motsi na narkewa sakamakon ƙaruwar ƙarancin yana haifar da manyan ajiyar carbon a cikin mantle.[85]
Dangane da haka, carbon na iya kasancewa a cikin ƙananan mantle na dogon lokaci, amma babban taro na carbon akai-akai yana samun hanyar komawa zuwa lithosphere. Wannan tsari, wanda ake kira carbon outgassing, shine sakamakon carbonated mantle dake fuskantar narkewar decompression, da kuma mantle plumes dauke da carbon mahadi zuwa ga ɓawon burodi.[86] Carbon yana narkewa a lokacin da yake hawa zuwa wuraren dake cike da wuta, inda aka sake shi azaman CO2. Wannan yana faruwa ne don atom din carbon yadace da yanayin oxidation na basalts dake fashewa a irin waɗannan yankuna.[87]

Carbon a cikin tsakiya
[gyara sashe | gyara masomin]Kodayake kasancewar carbon a cikin ainihin duniya yana da iyaka sosai, binciken da akayi kwanan nan ya nuna cewa za'a iya adana manyan kayayyaki na carbon a wannan yankin. [bayyanawa da ake buƙata] Raƙuman Shear (S) dake motsawa ta cikin tsakiya na ciki suna tafiya a kusan kashi hamsin cikin dari na saurin da ake tsammani ga mafi yawan ƙarfe masu wadata. Saboda anyi imanin cewa abun dake cikin core shine gami na ƙarfe mai haske da ƙaramin nickel, wannan anomaly na girgizar ƙasa yana nuna kasancewar abubuwa masu haske, gami da carbon, a cikin core. A zahiri, binciken da ke amfani da ƙwayoyin lu'u-lu'u don sake maimaita yanayin da ke cikin ainihin Duniya ya nuna cewa 7" href="./Cementite" id="mwAs0" rel="mw:WikiLink" title="Cementite">Karfe carbide (Fe7C3) ya dace da saurin raƙuman ruwa na ciki dayawa. Sabili da haka, samfurin ƙarfe na ƙarfe na iya zama shaida cewa ainihin yana riƙe da kashi 67% na carbon na Duniya.[88] Bugu da ƙari, wani binciken ya gano cewa a cikin matsin lamba da yanayin zafin jiki na ainihin duniya, carbon ya narke cikin baƙin ƙarfe kuma ya kafa wani lokaci mai ɗorewa tare da wannan abun dake ciki na Fe7 - duk da cewa yana da tsari daban daga wanda aka ambata a baya.[3] A taƙaice, kodayake ba'a san adadin carbon da za'a iya adanawa a cikin asalin Duniya ba, binciken da akayi kwanan nan ya nuna cewa kasancewar ƙarfe na ƙarfe na iya bayyana wasu abubuwan lura na geophysical.
Tasirin ɗan adam akan sake zagayowar carbon mai sauri
[gyara sashe | gyara masomin]
Tun lokacin juyin juya halin masana'antu, kuma musamman tun ƙarshen WWII, aikin ɗan adam ya dame sake zagayowar carbon na duniya ta hanyar sake rarraba yawan carbon daga geosphere.[1] Mutane sun kuma ci gaba da canza ayyukan sassan halitta na yanayin halittu na duniya tare da canje-canje ga ciyayi da sauran amfani da ƙasa.[5] An tsara mahaɗan carbon da aka yi da mutum (na roba) kuma anyi su dayawa wanda zai cigaba da shekaru dayawa zuwa dubban shekaru a cikin iska, ruwa, da turɓaya a matsayin gurɓataccen.[89] Canjin yanayi yana fadadawa kuma yana tilasta ƙarin canje-canje na ɗan adam kai tsaye zuwa sake zagayowar carbon a matsayin sakamakon ra'ayoyi masu kyau da marasa kyau.[21]
Canjin yanayi
[gyara sashe | gyara masomin]

Halin da ke faruwa a yanzu a cikin canjin yanayi yana haifar da yanayin zafi na teku da acidity, don haka yana canza yanayin halittu na ruwa.[91] Har ila yau, ruwan sama mai zafi da gurɓataccen ruwa daga aikin gona da masana'antu suna canza sinadarin sinadarai na teku. Irin waɗannan canje-canje na iya samun tasiri mai ban mamaki a kan yanayin halittu masu mahimmanci kamar coral reefs, [92] don haka iyakance ikon teku na sha carbon daga yanayi a kan sikelin yanki da rage bambancin halittu na teku a duniya.
Canjin carbon tsakanin yanayi da sauran abubuwan dake cikin tsarin duniya, wanda akafi sani da sake zagayowar carbon, a halin yanzu ya zama muhimmiyar mummunan ra'ayi (dampening) game da tasirin hayakin carbon na mutum akan canjin yanayi. Carbon nutsewa a cikin ƙasa da teku kowannensu a halin yanzu yana ɗaukar kusan kashi ɗaya cikin huɗu na hayakin carbon na mutum a kowace shekara.[93][90]
Ana sa ran waɗannan abubuwan zasu raunana a nan gaba, suna kara tasirin hayakin carbon na mutum akan canjin yanayi. Matsayin da zasu raunana, duk da haka, ba shi da tabbas, tare da samfuran tsarin Duniya da ke hasashen kewayon ƙasa da teku carbon ko da a ƙarƙashin maida hankali ga yanayi ko yanayin fitarwa.[94][90] Yankin Arctic methane wanda ya haifar da dumama na duniya ya shafi sake zagayowar carbon kuma ya ba da gudummawa ga cigaba da dumama.
Cire burbushin carbon da ƙonewa
[gyara sashe | gyara masomin]
Mafi girma kuma daya daga cikin tasirin mutum mafi sauri a kan sake zagayowar carbon da biosphere shine cirewa da ƙone burbushin burbushin halittu, wanda ke canja wurin carbon kai tsaye daga geosphere zuwa cikin yanayi. Hakanan ana samar da carbon dioxide kuma ana fitar dashi yayin ƙonewa dutse don samar da clinker. Clinker shine mai gabatarwa da masana'antu na siminti.
EatAs of 2020[update], an cire kimanin 450 gigatons na burbushin carbon gabaɗaya; adadin dake kusa da carbon dake ƙunshe a duk abubuwan dake rayuwa a duniya.[95] Yawan fitar da hayaki na duniya kai tsaye a cikin yanayi ya wuce abin da shuke-shuke da tekuna suka ɗauka.[96][97] Ana sa ran waɗannan sinks kuma an lura dasu don cire kusan rabin ƙarawar carbon a cikin kimanin ƙarni guda.[95] Duk da haka, sinks kamar teku suna da abubuwan dake tattare dasu, kuma wani bangare mai yawa (20-35%, bisa ga samfuran da aka haɗa) na carbon da aka kara ana sa ran zai kasance a cikin yanayi na ƙarni zuwa dubban shekaru. [98][99]
Halocarbons
[gyara sashe | gyara masomin]Halocarbons sune ƙananan mahadi waɗanda aka haɓaka don amfani daban-daban a duk masana'antu; misali a matsayin masu narkewa da masu sanyaya. Duk da haka, tara ƙananan ƙananan maida hankali (kashi na tiriliyan) na chlorofluorocarbon, hydrofluorocarbon. da iskar gas a cikin yanayi suna da alhakin kusan 10% na jimlar tilasta radiative kai tsaye daga duk iskar gas mai ɗorewa (shekara ta 2019); wanda ya haɗa da tilasta daga mafi girma na carbon dioxide da methane.[100] Chlorofluorocarbons kuma suna haifar da raguwar ozone na stratospheric. Ƙoƙarin ƙasashen duniya suna ci gaba a ƙarƙashin Yarjejeniyar Montreal da Yarjejeniyar Kyoto don sarrafa saurin cigaba a cikin masana'antu da amfani da waɗannan iskar gas masu ƙarfi. Ga wasu aikace-aikace an haɓaka wasu hanyoyin da basu da kyau kamar hydrofluoroolefins kuma ana gabatar da su a hankali.
Canjin amfani da ƙasa
[gyara sashe | gyara masomin]Tun lokacin da aka kirkiro aikin gona, mutane sun kai tsaye kuma a hankali sun rinjayi sake zagayowar carbon a tsawon lokaci ta hanyar canza cakuda ciyayi a cikin yanayin halittu na duniya. A cikin ƙarni da yawa da suka gabata, Amfani da ƙasa kai tsaye da kai tsaye da kuma sauyawar rufe ƙasa (LUCC) ya haifar da asarar bambancin halittu, wanda ke rage ƙarfin yanayin halittu ga matsalolin muhalli kuma yana rage ikon su na cire carbon daga yanayi. Fiye da haka, sau dayawa yana haifar da sakin carbon daga yanayin halittu na ƙasa zuwa cikin yanayi.
Kashe gandun daji don dalilai na noma yana cire gandun daji, wanda ke riƙe da adadi mai yawa na carbon, kuma ya maye gurbin su, gabaɗaya tare da aikin gona ko birane. Dukkanin waɗannan nau'ikan murfin ƙasa suna adana ƙananan carbon don sakamakon canjin shine ƙarin carbon ya kasance a cikin yanayi. Koyaya, tasirin dake kan yanayi da sake zagayowar carbon gabaɗaya ana iya juyawa da gangan da / ko kuma ta halitta tare da sake dasa bishiyoyi.
Dubi kuma
[gyara sashe | gyara masomin]
Manazarta
[gyara sashe | gyara masomin]- ↑ 1.0 1.1 1.2 Riebeek, Holli (16 June 2011). "The Carbon Cycle". Earth Observatory. NASA. Archived from the original on 5 March 2016. Retrieved 5 April 2018.
- ↑ 2.0 2.1 2.2 (Juan Luis ed.). Missing or empty
|title=(help) - ↑ 3.0 3.1 "The NOAA Annual Greenhouse Gas Index (AGGI) - An Introduction". NOAA Global Monitoring Laboratory/Earth System Research Laboratories. Retrieved 2020-10-30.
- ↑ "What is Ocean Acidification?". National Ocean Service, National Oceanic and Atmospheric Administration. Retrieved 2020-10-30.
- ↑ 5.0 5.1 5.2 5.3 5.4 5.5 5.6 Falkowski, P.; Scholes, R. J.; Boyle, E.; Canadell, J.; Canfield, D.; Elser, J.; Gruber, N.; Hibbard, K.; Högberg, P.; Linder, S.; MacKenzie, F. T.; Moore, III, B.; Pedersen, T.; Rosenthal, Y.; Seitzinger, S. (2000). "The Global Carbon Cycle: A Test of Our Knowledge of Earth as a System". Science. 290 (5490): 291–296. Bibcode:2000Sci...290..291F. doi:10.1126/science.290.5490.291. PMID 11030643.
- ↑ "An Introduction to the Global Carbon Cycle" (PDF). University of New Hampshire. 2009. Archived (PDF) from the original on 8 October 2016. Retrieved 6 February 2016.
- ↑ "A Year In The Life Of Earth's CO2" (Press release). NASA's Goddard Space Flight Center. 17 November 2014.
- ↑ Forster, P.; Ramawamy, V.; Artaxo, P.; Berntsen, T.; Betts, R.; Fahey, D.W.; Haywood, J.; Lean, J.; Lowe, D.C.; Myhre, G.; Nganga, J.; Prinn, R.; Raga, G.; Schulz, M.; Van Dorland, R. (2007). "Changes in atmospheric constituents and in radiative forcing". Climate Change 2007: The Physical Basis. Contribution of Working Group I to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change.
- ↑ "Many Planets, One Earth // Section 4: Carbon Cycling and Earth's Climate". Many Planets, One Earth. 4. Archived from the original on 17 April 2012. Retrieved 2012-06-24.
- ↑ 10.0 10.1 O'Malley-James, Jack T.; Greaves, Jane S.; Raven, John A.; Cockell, Charles S. (2012). "Swansong Biospheres: Refuges for life and novel microbial biospheres on terrestrial planets near the end of their habitable lifetimes". International Journal of Astrobiology. 12 (2): 99–112. arXiv:1210.5721. Bibcode:2013IJAsB..12...99O. doi:10.1017/S147355041200047X. S2CID 73722450.
- ↑ Walker, James C. G.; Hays, P. B.; Kasting, J. F. (20 October 1981). "A negative feedback mechanism for the long-term stabilization of Earth's surface temperature". Journal of Geophysical Research: Oceans. 86 (C10): 9776–9782. Bibcode:1981JGR....86.9776W. doi:10.1029/JC086iC10p09776.
- ↑ Crockford, Peter W.; Bar On, Yinon M.; Ward, Luce M.; Milo, Ron; Halevy, Itay (November 2023). "The geologic history of primary productivity". Current Biology. 33 (21): 4741–4750.e5. Bibcode:2023CBio...33E4741C. doi:10.1016/j.cub.2023.09.040. PMID 37827153 Check
|pmid=value (help). - ↑ Cite error: Invalid
<ref>tag; no text was provided for refs named:1 - ↑ Lenton, Timothy M.; von Bloh, Werner (May 2001). "Biotic feedback extends the life span of the biosphere". Geophysical Research Letters. 28 (9): 1715–1718. Bibcode:2001GeoRL..28.1715L. doi:10.1029/2000GL012198.
- ↑ (George L. ed.). Missing or empty
|title=(help) - ↑ Rice, Charles W. (January 2002). "Storing carbon in soil: Why and how?". Geotimes. 47 (1): 14–17. Archived from the original on 5 April 2018. Retrieved 5 April 2018.
- ↑ Yousaf, Balal; Liu, Guijian; Wang, Ruwei; Abbas, Qumber; Imtiaz, Muhammad; Liu, Ruijia (2016). "Investigating the biochar effects on C-mineralization and sequestration of carbon in soil compared with conventional amendments using the stable isotope (δ13C) approach". GCB Bioenergy. 9 (6): 1085–1099. doi:10.1111/gcbb.12401.
- ↑ Lal, Rattan (2008). "Sequestration of atmospheric CO2 in global carbon pools". Energy and Environmental Science. 1: 86–100. doi:10.1039/b809492f.
- ↑ Li, Mingxu; Peng, Changhui; Wang, Meng; Xue, Wei; Zhang, Kerou; Wang, Kefeng; Shi, Guohua; Zhu, Qiuan (2017). "The carbon flux of global rivers: A re-evaluation of amount and spatial patterns". Ecological Indicators. 80: 40–51. Bibcode:2017EcInd..80...40L. doi:10.1016/j.ecolind.2017.04.049.
- ↑ Bond-Lamberty, Ben; Thomson, Allison (2010). "Temperature-associated increases in the global soil respiration record". Nature. 464 (7288): 579–582. Bibcode:2010Natur.464..579B. doi:10.1038/nature08930. PMID 20336143. S2CID 4412623.
- ↑ 21.0 21.1 Varney, Rebecca M.; Chadburn, Sarah E.; Friedlingstein, Pierre; Burke, Eleanor J.; Koven, Charles D.; Hugelius, Gustaf; Cox, Peter M. (2 November 2020). "A spatial emergent constraint on the sensitivity of soil carbon turnover to global warming". Nature Communications. 11 (1): 5544. Bibcode:2020NatCo..11.5544V. doi:10.1038/s41467-020-19208-8. PMC 7608627. PMID 33139706.
- ↑ Kleypas, J. A.; Buddemeier, R. W.; Archer, D.; Gattuso, J. P.; Langdon, C.; Opdyke, B. N. (1999). "Geochemical Consequences of Increased Atmospheric Carbon Dioxide on Coral Reefs". Science. 284 (5411): 118–120. Bibcode:1999Sci...284..118K. doi:10.1126/science.284.5411.118. PMID 10102806.
- ↑ Langdon, C.; Takahashi, T.; Sweeney, C.; Chipman, D.; Goddard, J.; Marubini, F.; Aceves, H.; Barnett, H.; Atkinson, M. J. (2000). "Effect of calcium carbonate saturation state on the calcification rate of an experimental coral reef". Global Biogeochemical Cycles. 14 (2): 639. Bibcode:2000GBioC..14..639L. doi:10.1029/1999GB001195. S2CID 128987509.
- ↑ Berner, Robert A. (November 1999). "A New Look at the Long-term Carbon Cycle" (PDF). GSA Today. 9 (11): 1–6. Archived (PDF) from the original on 2019-02-13.
- ↑ "The Slow Carbon Cycle". NASA. 2011-06-16. Archived from the original on 16 June 2012. Retrieved 2012-06-24.
- ↑ Rothman, D. H. (2002). "Atmospheric carbon dioxide levels for the last 500 million years". Proceedings of the National Academy of Sciences. 99 (7): 4167–4171. Bibcode:2002PNAS...99.4167R. doi:10.1073/pnas.022055499. PMC 123620. PMID 11904360.
- ↑ Carpinteri, Alberto; Niccolini, Gianni (2019). "Correlation between the Fluctuations in Worldwide Seismicity and Atmospheric Carbon Pollution". Sci. 1: 17. doi:10.3390/sci1010017.
- ↑ Rothman, Daniel H. (17 September 2014). "Earth's carbon cycle: A mathematical perspective". Bulletin of the American Mathematical Society. 52 (1): 47–64. doi:10.1090/S0273-0979-2014-01471-5.
|hdl-access=requires|hdl=(help) - ↑ NASA Earth Observatory (16 June 2011). "The Slow Carbon Cycle". Archive.
This article incorporates text from this source, which is in the public domain.
- ↑ Ward, Nicholas D.; Bianchi, Thomas S.; Medeiros, Patricia M.; Seidel, Michael; Richey, Jeffrey E.; Keil, Richard G.; Sawakuchi, Henrique O. (31 January 2017). "Where Carbon Goes When Water Flows: Carbon Cycling across the Aquatic Continuum". Frontiers in Marine Science. 4. doi:10.3389/fmars.2017.00007.
- ↑ Kerminen, Veli-Matti; Virkkula, Aki; Hillamo, Risto; Wexler, Anthony S.; Kulmala, Markku (16 April 2000). "Secondary organics and atmospheric cloud condensation nuclei production". Journal of Geophysical Research: Atmospheres. 105 (D7): 9255–9264. Bibcode:2000JGR...105.9255K. doi:10.1029/1999JD901203.
- ↑ Riipinen, I.; Pierce, J. R.; Yli-Juuti, T.; Nieminen, T.; Häkkinen, S.; Ehn, M.; Junninen, H.; Lehtipalo, K.; Petäjä, T.; Slowik, J.; Chang, R.; Shantz, N. C.; Abbatt, J.; Leaitch, W. R.; Kerminen, V.-M. (27 April 2011). "Organic condensation: a vital link connecting aerosol formation to cloud condensation nuclei (CCN) concentrations". Atmospheric Chemistry and Physics. 11 (8): 3865–3878. Bibcode:2011ACP....11.3865R. doi:10.5194/acp-11-3865-2011.
- ↑ Waterloo, Maarten J.; Oliveira, Sylvia M.; Drucker, Debora P.; Nobre, Antonio D.; Cuartas, Luz A.; Hodnett, Martin G.; Langedijk, Ivar; Jans, Wilma W. P.; Tomasella, Javier; de Araújo, Alessandro C.; Pimentel, Tania P.; Múnera Estrada, Juan C. (15 August 2006). "Export of organic carbon in run-off from an Amazonian rainforest blackwater catchment". Hydrological Processes. 20 (12): 2581–2597. Bibcode:2006HyPr...20.2581W. doi:10.1002/hyp.6217.
- ↑ Neu, Vania; Ward, Nicholas D.; Krusche, Alex V.; Neill, Christopher (28 June 2016). "Dissolved Organic and Inorganic Carbon Flow Paths in an Amazonian Transitional Forest". Frontiers in Marine Science. 3. doi:10.3389/fmars.2016.00114.
- ↑ Baldock, J.A.; Masiello, C.A.; Gélinas, Y.; Hedges, J.I. (December 2004). "Cycling and composition of organic matter in terrestrial and marine ecosystems". Marine Chemistry. 92 (1–4): 39–64. Bibcode:2004MarCh..92...39B. doi:10.1016/j.marchem.2004.06.016.
- ↑ Myers-Pigg, Allison N.; Griffin, Robert J.; Louchouarn, Patrick; Norwood, Matthew J.; Sterne, Amanda; Cevik, Basak Karakurt (6 September 2016). "Signatures of Biomass Burning Aerosols in the Plume of a Saltmarsh Wildfire in South Texas". Environmental Science & Technology. 50 (17): 9308–9314. Bibcode:2016EnST...50.9308M. doi:10.1021/acs.est.6b02132. PMID 27462728.
- ↑ Field, Christopher B.; Behrenfeld, Michael J.; Randerson, James T.; Falkowski, Paul (10 July 1998). "Primary Production of the Biosphere: Integrating Terrestrial and Oceanic Components". Science. 281 (5374): 237–240. Bibcode:1998Sci...281..237F. doi:10.1126/science.281.5374.237. PMID 9657713.
- ↑ Martens, Dean A.; Reedy, Thomas E.; Lewis, David T. (January 2004). "Soil organic carbon content and composition of 130-year crop, pasture and forest land-use managements". Global Change Biology. 10 (1): 65–78. Bibcode:2004GCBio..10...65M. doi:10.1046/j.1529-8817.2003.00722.x.
- ↑ Bose, Samar K.; Francis, Raymond C.; Govender, Mark; Bush, Tamara; Spark, Andrew (February 2009). "Lignin content versus syringyl to guaiacyl ratio amongst poplars". Bioresource Technology. 100 (4): 1628–1633. Bibcode:2009BiTec.100.1628B. doi:10.1016/j.biortech.2008.08.046. PMID 18954979.
- ↑ Schlesinger, William H.; Andrews, Jeffrey A. (2000). "Soil respiration and the global carbon cycle". Biogeochemistry. 48 (1): 7–20. doi:10.1023/A:1006247623877.
- ↑ Schmidt, Michael W. I.; Torn, Margaret S.; Abiven, Samuel; Dittmar, Thorsten; Guggenberger, Georg; Janssens, Ivan A.; Kleber, Markus; Kögel-Knabner, Ingrid; Lehmann, Johannes; Manning, David A. C.; Nannipieri, Paolo; Rasse, Daniel P.; Weiner, Steve; Trumbore, Susan E. (October 2011). "Persistence of soil organic matter as an ecosystem property". Nature. 478 (7367): 49–56. Bibcode:2011Natur.478...49S. doi:10.1038/nature10386. PMID 21979045.
- ↑ Lehmann, Johannes; Kleber, Markus (December 2015). "The contentious nature of soil organic matter". Nature. 528 (7580): 60–68. Bibcode:2015Natur.528...60L. doi:10.1038/nature16069. PMID 26595271.
- ↑ Qualls, Robert G.; Haines, Bruce L. (March 1992). "Biodegradability of Dissolved Organic Matter in Forest Throughfall, Soil Solution, and Stream Water". Soil Science Society of America Journal. 56 (2): 578–586. Bibcode:1992SSASJ..56..578Q. doi:10.2136/sssaj1992.03615995005600020038x.
- ↑ Grøn, Christian; Tørsløv, Jens; Albrechtsen, Hans-Jørgen; Jensen, Hanne Møller (May 1992). "Biodegradability of dissolved organic carbon in groundwater from an unconfined aquifer". Science of the Total Environment. 117-118: 241–251. Bibcode:1992ScTEn.117..241G. doi:10.1016/0048-9697(92)90091-6.
- ↑ Pabich, Wendy J.; Valiela, Ivan; Hemond, Harold F. (2001). "Relationship between DOC concentration and vadose zone thickness and depth below water table in groundwater of Cape Cod, U.S.A.". Biogeochemistry. 55 (3): 247–268. doi:10.1023/A:1011842918260.
- ↑ Horton, Robert E. (June 1933). "The Rôle of infiltration in the hydrologic cycle". Eos, Transactions American Geophysical Union. 14 (1): 446–460. Bibcode:1933TrAGU..14..446H. doi:10.1029/TR014i001p00446.
- ↑ Richey, Jeffrey E.; Melack, John M.; Aufdenkampe, Anthony K.; Ballester, Victoria M.; Hess, Laura L. (April 2002). "Outgassing from Amazonian rivers and wetlands as a large tropical source of atmospheric CO2". Nature. 416 (6881): 617–620. doi:10.1038/416617a. PMID 11948346.
- ↑ Cole, J. J.; Prairie, Y. T.; Caraco, N. F.; McDowell, W. H.; Tranvik, L. J.; Striegl, R. G.; Duarte, C. M.; Kortelainen, P.; Downing, J. A.; Middelburg, J. J.; Melack, J. (February 2007). "Plumbing the Global Carbon Cycle: Integrating Inland Waters into the Terrestrial Carbon Budget". Ecosystems. 10 (1): 172–185. Bibcode:2007Ecosy..10..172C. doi:10.1007/s10021-006-9013-8.
- ↑ 49.0 49.1 Raymond, Peter A.; Hartmann, Jens; Lauerwald, Ronny; Sobek, Sebastian; McDonald, Cory; Hoover, Mark; Butman, David; Striegl, Robert; Mayorga, Emilio; Humborg, Christoph; Kortelainen, Pirkko; Dürr, Hans; Meybeck, Michel; Ciais, Philippe; Guth, Peter (21 November 2013). "Global carbon dioxide emissions from inland waters". Nature. 503 (7476): 355–359. Bibcode:2013Natur.503..355R. doi:10.1038/nature12760. PMID 24256802.
- ↑ Ward, Nicholas D.; Keil, Richard G.; Medeiros, Patricia M.; Brito, Daimio C.; Cunha, Alan C.; Dittmar, Thorsten; Yager, Patricia L.; Krusche, Alex V.; Richey, Jeffrey E. (July 2013). "Degradation of terrestrially derived macromolecules in the Amazon River". Nature Geoscience. 6 (7): 530–533. Bibcode:2013NatGe...6..530W. doi:10.1038/ngeo1817.
- ↑ Myers-Pigg, Allison N.; Louchouarn, Patrick; Amon, Rainer M. W.; Prokushkin, Anatoly; Pierce, Kayce; Rubtsov, Alexey (28 January 2015). "Labile pyrogenic dissolved organic carbon in major Siberian Arctic rivers: Implications for wildfire-stream metabolic linkages". Geophysical Research Letters. 42 (2): 377–385. Bibcode:2015GeoRL..42..377M. doi:10.1002/2014GL062762.
- ↑ Tranvik, Lars J.; Downing, John A.; Cotner, James B.; Loiselle, Steven A.; Striegl, Robert G.; Ballatore, Thomas J.; Dillon, Peter; Finlay, Kerri; Fortino, Kenneth; Knoll, Lesley B.; Kortelainen, Pirkko L.; Kutser, Tiit; Larsen, Soren.; Laurion, Isabelle; Leech, Dina M. (November 2009). "Lakes and reservoirs as regulators of carbon cycling and climate". Limnology and Oceanography. 54 (6part2): 2298–2314. Bibcode:2009LimOc..54.2298T. doi:10.4319/lo.2009.54.6_part_2.2298.
- ↑ Bastviken, David; Cole, Jonathan; Pace, Michael; Tranvik, Lars (December 2004). "Methane emissions from lakes: Dependence of lake characteristics, two regional assessments, and a global estimate". Global Biogeochemical Cycles. 18 (4). Bibcode:2004GBioC..18.4009B. doi:10.1029/2004GB002238.
- ↑ Cooley, S. R.; Coles, V. J.; Subramaniam, A.; Yager, P. L. (September 2007). "Seasonal variations in the Amazon plume-related atmospheric carbon sink". Global Biogeochemical Cycles. 21 (3). Bibcode:2007GBioC..21.3014C. doi:10.1029/2006GB002831.
- ↑ Subramaniam, A.; Yager, P. L.; Carpenter, E. J.; Mahaffey, C.; Björkman, K.; Cooley, S.; Kustka, A. B.; Montoya, J. P.; Sañudo-Wilhelmy, S. A.; Shipe, R.; Capone, D. G. (29 July 2008). "Amazon River enhances diazotrophy and carbon sequestration in the tropical North Atlantic Ocean". Proceedings of the National Academy of Sciences. 105 (30): 10460–10465. doi:10.1073/pnas.0710279105. PMC 2480616. PMID 18647838.
- ↑ 56.0 56.1 Cai, Wei-Jun (15 January 2011). "Estuarine and Coastal Ocean Carbon Paradox: CO 2 Sinks or Sites of Terrestrial Carbon Incineration?". Annual Review of Marine Science. 3 (1): 123–145. Bibcode:2011ARMS....3..123C. doi:10.1146/annurev-marine-120709-142723. PMID 21329201.
- ↑ Dittmar, Thorsten; Lara, Rubén José; Kattner, Gerhard (March 2001). "River or mangrove? Tracing major organic matter sources in tropical Brazilian coastal waters". Marine Chemistry. 73 (3–4): 253–271. Bibcode:2001MarCh..73..253D. doi:10.1016/s0304-4203(00)00110-9.
- ↑ Moore, W.S.; Beck, M.; Riedel, T.; Rutgers van der Loeff, M.; Dellwig, O.; Shaw, T.J.; Schnetger, B.; Brumsack, H.-J. (November 2011). "Radium-based pore water fluxes of silica, alkalinity, manganese, DOC, and uranium: A decade of studies in the German Wadden Sea". Geochimica et Cosmochimica Acta. 75 (21): 6535–6555. Bibcode:2011GeCoA..75.6535M. doi:10.1016/j.gca.2011.08.037.
- ↑ Wehrli, Bernhard (November 2013). "Conduits of the carbon cycle". Nature. 503 (7476): 346–347. doi:10.1038/503346a. PMID 24256800.
- ↑ Moran, Mary Ann; Kujawinski, Elizabeth B.; Stubbins, Aron; Fatland, Rob; Aluwihare, Lihini I.; Buchan, Alison; Crump, Byron C.; Dorrestein, Pieter C.; Dyhrman, Sonya T.; Hess, Nancy J.; Howe, Bill; Longnecker, Krista; Medeiros, Patricia M.; Niggemann, Jutta; Obernosterer, Ingrid (22 March 2016). "Deciphering ocean carbon in a changing world". Proceedings of the National Academy of Sciences. 113 (12): 3143–3151. Bibcode:2016PNAS..113.3143M. doi:10.1073/pnas.1514645113. PMC 4812754. PMID 26951682.
- ↑ 61.0 61.1 61.2 Gao, Yang; Jia, Junjie; Lu, Yao; Sun, Kun; Wang, Jing; Wang, Shuoyue (2022). "Carbon transportation, transformation, and sedimentation processes at the land-river-estuary continuum". Fundamental Research. Elsevier BV. doi:10.1016/j.fmre.2022.07.007. S2CID 251168582 Check
|s2cid=value (help). - ↑ Schlünz, B.; Schneider, R. R. (2000-03-22). "Transport of terrestrial organic carbon to the oceans by rivers: re-estimating flux- and burial rates". International Journal of Earth Sciences. Springer Science and Business Media LLC. 88 (4): 599–606. Bibcode:2000IJEaS..88..599S. doi:10.1007/s005310050290. S2CID 128411658.
- ↑ Blair, Neal E.; Leithold, Elana L.; Aller, Robert C. (2004). "From bedrock to burial: The evolution of particulate organic carbon across coupled watershed-continental margin systems". Marine Chemistry. 92 (1–4): 141–156. Bibcode:2004MarCh..92..141B. doi:10.1016/j.marchem.2004.06.023.
- ↑ Bouchez, Julien; Beyssac, Olivier; Galy, Valier; Gaillardet, Jérôme; France-Lanord, Christian; Maurice, Laurence; Moreira-Turcq, Patricia (2010). "Oxidation of petrogenic organic carbon in the Amazon floodplain as a source of atmospheric CO2". Geology. Geological Society of America. 38 (3): 255–258. Bibcode:2010Geo....38..255B. doi:10.1130/g30608.1. S2CID 53512466.
- ↑ Regnier, Pierre; Friedlingstein, Pierre; Ciais, Philippe; Mackenzie, Fred T.; Gruber, Nicolas; Janssens, Ivan A.; Laruelle, Goulven G.; Lauerwald, Ronny; Luyssaert, Sebastiaan; Andersson, Andreas J.; Arndt, Sandra; Arnosti, Carol; Borges, Alberto V.; Dale, Andrew W.; Gallego-Sala, Angela (August 2013). "Anthropogenic perturbation of the carbon fluxes from land to ocean". Nature Geoscience. 6 (8): 597–607. Bibcode:2013NatGe...6..597R. doi:10.1038/ngeo1830.
- ↑ 66.0 66.1 Bauer, James E.; Cai, Wei-Jun; Raymond, Peter A.; Bianchi, Thomas S.; Hopkinson, Charles S.; Regnier, Pierre A. G. (5 December 2013). "The changing carbon cycle of the coastal ocean". Nature. 504 (7478): 61–70. Bibcode:2013Natur.504...61B. doi:10.1038/nature12857. PMID 24305149. S2CID 4399374.
- ↑ Cai, Wei-Jun (15 January 2011). "Estuarine and Coastal Ocean Carbon Paradox: CO 2 Sinks or Sites of Terrestrial Carbon Incineration?". Annual Review of Marine Science. 3 (1): 123–145. Bibcode:2011ARMS....3..123C. doi:10.1146/annurev-marine-120709-142723. PMID 21329201.
- ↑ Galy, Valier; Peucker-Ehrenbrink, Bernhard; Eglinton, Timothy (May 2015). "Global carbon export from the terrestrial biosphere controlled by erosion". Nature. 521 (7551): 204–207. Bibcode:2015Natur.521..204G. doi:10.1038/nature14400. PMID 25971513. S2CID 205243485.
- ↑ Sigman DM & GH Haug. 2006. The biological pump in the past. In: Treatise on Geochemistry; vol. 6, (ed.). Pergamon Press, pp. 491–528
- ↑ Sanders, Richard; Henson, Stephanie A.; Koski, Marja; De La Rocha, Christina L.; Painter, Stuart C.; Poulton, Alex J.; Riley, Jennifer; Salihoglu, Baris; Visser, Andre; Yool, Andrew; Bellerby, Richard; Martin, Adrian P. (December 2014). "The Biological Carbon Pump in the North Atlantic". Progress in Oceanography. 129: 200–218. Bibcode:2014PrOce.129..200S. doi:10.1016/j.pocean.2014.05.005.
- ↑ Boyd, Philip W. (13 October 2015). "Toward quantifying the response of the oceans' biological pump to climate change". Frontiers in Marine Science. 2. doi:10.3389/fmars.2015.00077.
- ↑ Basu, Samarpita; Mackey, Katherine (19 March 2018). "Phytoplankton as Key Mediators of the Biological Carbon Pump: Their Responses to a Changing Climate". Sustainability. 10 (3): 869. doi:10.3390/su10030869.
- ↑ Steinberg, Deborah K; Goldthwait, Sarah A; Hansell, Dennis A (August 2002). "Zooplankton vertical migration and the active transport of dissolved organic and inorganic nitrogen in the Sargasso Sea". Deep Sea Research Part I: Oceanographic Research Papers. 49 (8): 1445–1461. Bibcode:2002DSRI...49.1445S. doi:10.1016/S0967-0637(02)00037-7.
- ↑ 74.0 74.1 Ducklow, Hugh; Steinberg, Deborah; Buesseler, Ken (2001). "Upper Ocean Carbon Export and the Biological Pump". Oceanography. 14 (4): 50–58. doi:10.5670/oceanog.2001.06.
- ↑ Wong, Kevin; Mason, Emily; Brune, Sascha; East, Madison; Edmonds, Marie; Zahirovic, Sabin (11 October 2019). "Deep Carbon Cycling Over the Past 200 Million Years: A Review of Fluxes in Different Tectonic Settings". Frontiers in Earth Science. 7: 263. Bibcode:2019FrEaS...7..263W. doi:10.3389/feart.2019.00263.
- ↑ Wilson, Mark (2003). "Where do Carbon Atoms Reside within Earth's Mantle?". Physics Today. 56 (10): 21–22. Bibcode:2003PhT....56j..21W. doi:10.1063/1.1628990.
- ↑ Dasgupta, Rajdeep (10 December 2011). "From Magma Ocean to Crustal Recycling: Earth's Deep Carbon Cycle". Archived from the original on 24 April 2016. Retrieved 9 March 2019.
- ↑ Stagno, V.; Frost, D. J.; McCammon, C. A.; Mohseni, H.; Fei, Y. (February 2015). "The oxygen fugacity at which graphite or diamond forms from carbonate-bearing melts in eclogitic rocks". Contributions to Mineralogy and Petrology. 169 (2): 16. Bibcode:2015CoMP..169...16S. doi:10.1007/s00410-015-1111-1.
- ↑ Boulard, Eglantine; Gloter, Alexandre; Corgne, Alexandre; Antonangeli, Daniele; Auzende, Anne-Line; Perrillat, Jean-Philippe; Guyot, François; Fiquet, Guillaume (29 March 2011). "New host for carbon in the deep Earth". Proceedings of the National Academy of Sciences. 108 (13): 5184–5187. Bibcode:2011PNAS..108.5184B. doi:10.1073/pnas.1016934108. PMC 3069163. PMID 21402927.
- ↑ Dorfman, Susannah M.; Badro, James; Nabiei, Farhang; Prakapenka, Vitali B.; Cantoni, Marco; Gillet, Philippe (May 2018). "Carbonate stability in the reduced lower mantle". Earth and Planetary Science Letters. 489: 84–91. Bibcode:2018E&PSL.489...84D. doi:10.1016/j.epsl.2018.02.035.
- ↑ Cottrell, Elizabeth; Kelley, Katherine A. (14 June 2013). "Redox Heterogeneity in Mid-Ocean Ridge Basalts as a Function of Mantle Source". Science. 340 (6138): 1314–1317. Bibcode:2013Sci...340.1314C. doi:10.1126/science.1233299. PMID 23641060.
- ↑ (Chrystèle ed.). Missing or empty
|title=(help)[page needed] - ↑ Boulard, Eglantine; Gloter, Alexandre; Corgne, Alexandre; Antonangeli, Daniele; Auzende, Anne-Line; Perrillat, Jean-Philippe; Guyot, François; Fiquet, Guillaume (29 March 2011). "New host for carbon in the deep Earth". Proceedings of the National Academy of Sciences. 108 (13): 5184–5187. Bibcode:2011PNAS..108.5184B. doi:10.1073/pnas.1016934108. PMC 3069163. PMID 21402927.
- ↑ Boulard, Eglantine; Pan, Ding; Galli, Giulia; Liu, Zhenxian; Mao, Wendy L. (18 February 2015). "Tetrahedrally coordinated carbonates in Earth's lower mantle". Nature Communications. 6 (1): 6311. arXiv:1503.03538. Bibcode:2015NatCo...6.6311B. doi:10.1038/ncomms7311. PMID 25692448.
- ↑ Jones, A. P.; Genge, M.; Carmody, L. (January 2013). "Carbonate Melts and Carbonatites". Reviews in Mineralogy and Geochemistry. 75 (1): 289–322. Bibcode:2013RvMG...75..289J. doi:10.2138/rmg.2013.75.10.
- ↑ Dasgupta, Rajdeep; Hirschmann, Marc M. (September 2010). "The deep carbon cycle and melting in Earth's interior". Earth and Planetary Science Letters. 298 (1–2): 1–13. Bibcode:2010E&PSL.298....1D. doi:10.1016/j.epsl.2010.06.039.
- ↑ Frost, Daniel J.; McCammon, Catherine A. (May 2008). "The Redox State of Earth's Mantle". Annual Review of Earth and Planetary Sciences. 36 (1): 389–420. Bibcode:2008AREPS..36..389F. doi:10.1146/annurev.earth.36.031207.124322.
- ↑ Chen, Bin; Li, Zeyu; Zhang, Dongzhou; Liu, Jiachao; Hu, Michael Y.; Zhao, Jiyong; Bi, Wenli; Alp, E. Ercan; Xiao, Yuming; Chow, Paul; Li, Jie (16 December 2014). "Hidden carbon in Earth's inner core revealed by shear softening in dense [[:Samfuri:Chem]]". Proceedings of the National Academy of Sciences. 111 (50): 17755–17758. Bibcode:2014PNAS..11117755C. doi:10.1073/pnas.1411154111. PMC 4273394. PMID 25453077. URL–wikilink conflict (help)
- ↑ "Overview of greenhouse gases". U.S. Environmental Protection Agency. 23 December 2015. Retrieved 2020-11-02.
- ↑ 90.0 90.1 90.2 Lade, Steven J.; Donges, Jonathan F.; Fetzer, Ingo; Anderies, John M.; Beer, Christian; Cornell, Sarah E.; Gasser, Thomas; Norberg, Jon; Richardson, Katherine; Rockström, Johan; Steffen, Will (2018). "Analytically tractable climate–carbon cycle feedbacks under 21st century anthropogenic forcing". Earth System Dynamics. 9 (2): 507–523. Bibcode:2018ESD.....9..507L. doi:10.5194/esd-9-507-2018.
|hdl-access=requires|hdl=(help) - ↑ Takahashi, Taro; Sutherland, Stewart C.; Sweeney, Colm; Poisson, Alain; Metzl, Nicolas; Tilbrook, Bronte; Bates, Nicolas; Wanninkhof, Rik; Feely, Richard A.; Sabine, Christopher; Olafsson, Jon; Nojiri, Yukihiro (2002). "Global sea–air CO2 flux based on climatological surface ocean pCO2, and seasonal biological and temperature effects". Deep Sea Research Part II: Topical Studies in Oceanography. 49 (9–10): 1601–1622. Bibcode:2002DSRII..49.1601T. doi:10.1016/S0967-0645(02)00003-6.
- ↑ Orr, James C.; Fabry, Victoria J.; Aumont, Olivier; Bopp, Laurent; Doney, Scott C.; Feely, Richard A.; Gnanadesikan, Anand; Gruber, Nicolas; Ishida, Akio; Joos, Fortunat; Key, Robert M.; Lindsay, Keith; Maier-Reimer, Ernst; Matear, Richard; Monfray, Patrick (September 2005). "Anthropogenic ocean acidification over the twenty-first century and its impact on calcifying organisms". Nature. 437 (7059): 681–686. Bibcode:2005Natur.437..681O. doi:10.1038/nature04095. PMID 16193043. S2CID 4306199.
- ↑ Le Quéré, Corinne; Andrew, Robbie M.; Canadell, Josep G.; Sitch, Stephen; Korsbakken, Jan Ivar; Peters, Glen P.; Manning, Andrew C.; Boden, Thomas A.; Tans, Pieter P.; Houghton, Richard A.; Keeling, Ralph F.; Alin, Simone; Andrews, Oliver D.; Anthoni, Peter; Barbero, Leticia (2016). "Global Carbon Budget 2016". Earth System Science Data. 8 (2): 605–649. Bibcode:2016ESSD....8..605L. doi:10.5194/essd-8-605-2016.
|hdl-access=requires|hdl=(help); Invalid|display-authors=29(help) - ↑ Joos, F.; Roth, R.; Fuglestvedt, J. S.; Peters, G. P.; Enting, I. G.; von Bloh, W.; Brovkin, V.; Burke, E. J.; Eby, M.; Edwards, N. R.; Friedrich, T.; Frölicher, T. L.; Halloran, P. R.; Holden, P. B.; Jones, C. (2013). "Carbon dioxide and climate impulse response functions for the computation of greenhouse gas metrics: A multi-model analysis". Atmospheric Chemistry and Physics. 13 (5): 2793–2825. Bibcode:2013ACP....13.2793J. doi:10.5194/acp-13-2793-2013.
|hdl-access=requires|hdl=(help) - ↑ 95.0 95.1 95.2 Friedlingstein, Pierre; Jones, Matthew W.; O'Sullivan, Michael; Andrew, Robbie M.; Hauck, Judith; Peters, Glen P.; Peters, Wouter; Pongratz, Julia; Sitch, Stephen; Le Quéré, Corinne; Bakker, Dorothee C. E.; Canadell, Josep G.; Ciais, Philippe; Jackson, Robert B.; Anthoni, Peter (4 December 2019). "Global Carbon Budget 2019". Earth System Science Data. 11 (4): 1783–1838. Bibcode:2019ESSD...11.1783F. doi:10.5194/essd-11-1783-2019.
|hdl-access=requires|hdl=(help) - ↑ Buis, Alan; Ramsayer, Kate; Rasmussen, Carol (12 November 2015). "A Breathing Planet, Off Balance". NASA. Archived from the original on 14 November 2015. Retrieved 13 November 2015.
- ↑ "Audio (66:01) - NASA News Conference - Carbon & Climate Telecon". NASA. 12 November 2015. Archived from the original on 17 November 2015. Retrieved 12 November 2015.
- ↑ Archer, David (2009). "Atmospheric lifetime of fossil fuel carbon dioxide". Annual Review of Earth and Planetary Sciences. 37 (1): 117–34. Bibcode:2009AREPS..37..117A. doi:10.1146/annurev.earth.031208.100206.
- ↑ Joos, F.; Roth, R.; Fuglestvedt, J.D.; et al. (2013). "Carbon dioxide and climate impulse response functions for the computation of greenhouse gas metrics: A multi-model analysis". Atmospheric Chemistry and Physics. 13 (5): 2793–2825. doi:10.5194/acpd-12-19799-2012.
|hdl-access=requires|hdl=(help) - ↑ Butler, J.; Montzka, S. (2020). "The NOAA Annual Greenhouse Gas Index (AGGI)". NOAA Global Monitoring Laboratory/Earth System Research Laboratories.
Haɗin waje
[gyara sashe | gyara masomin]- Shirin Kimiyya na Carbon Cycle - haɗin gwiwar hukuma.
- Kungiyar Gishiri ta NOAA
- Shirin Carbon na Duniya - shirin hadin gwiwar Kimiyya na Tsarin Duniya
- UNEP - Tsarin carbon na yanzu - Canjin Yanayi Archived 2008-09-15 at the Wayback Machine An adana shi matakan carbon da gudana
- NASA's Orbiting Carbon Observatory An adana shi
- Pages with reference errors
- Pages with citations lacking titles
- CS1 errors: PMID
- CS1 errors: param-access
- CS1: long volume value
- CS1 errors: S2CID
- Wikipedia articles needing page number citations from July 2024
- Articles with invalid date parameter in template
- CS1 errors: URL–wikilink conflict
- CS1 errors: display-names
- All articles with unsourced statements
- Articles with unsourced statements from April 2024
- Articles containing potentially dated statements from 2020
- All articles containing potentially dated statements
- Webarchive template wayback links
- Wikipedia articles with BNF identifiers
- Wikipedia articles with GND identifiers
- Wikipedia articles with LCCN identifiers
- Wikipedia articles with NKC identifiers
- Pages with red-linked authority control categories
- Wikipedia articles with SUDOC identifiers
- Muhalli
- Shafuka masu fassarorin da ba'a duba ba