Intramolecular reaction
Intramolecular in chemistry describes a process or characteristic limited within the structure of a single molecule, a property or phenomenon limited to the extent of a single molecule.
Examples
- intramolecular hydride transfer (transfer of a hydride ion from one part to another within the same molecule)
- intramolecular hydrogen bond (a hydrogen bond formed between two functional groups of the same molecule)
In intramolecular organic reactions, two reaction sites are contained within a single molecule. This creates a very high effective concentration (resulting in high reaction rates), and, therefore, many intramolecular reactions that would not occur as an intermolecular reaction between two compounds take place.
Examples of intramolecular reactions are the Smiles rearrangement, the Dieckmann condensation and the Madelung synthesis.
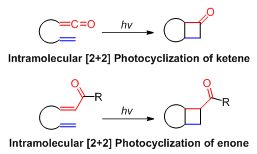
Tethered Intramolecular [2+2] Reactions
Tethered Intramolecular [2+2] reactions entail the formation of four-membered rings from two functional groups in the same molecule, each functional group contributes two atoms. These two functional groups are tethered by a carbon chain, hence the intramolecular [2+2] reaction usually forms a multi-cyclic system, which is an important carbon skeleton structure in organic chemistry.
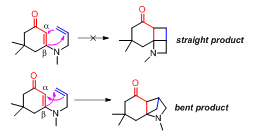
The length of the tether effects the stereochemical outcome of the [2+2] reaction. Longer tethers tend to generate the “straight” product where the terminal carbon of the alkene is linked to the α-carbon of the enone.[1] When the tether consists only two carbons, the “bent” product is generated where the β-carbon of the enone is connected to the terminal carbon of the alkene (Figure 2).[2]
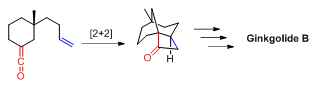
Tethered [2+2] reactions have been used to synthesize organic compounds with interesting ring systems and topologies. For example, [2+2] photocyclization was used to construct the tricyclic core structure in Ginkgolide B by E. J. Corey and co-workers[3] in 1988.
Molecular tethers
In a niche concept called molecular tethers, otherwise-intermolecular reactions can be made temporarily intramolecular by anchoring both reactions by a tether with all the advantages associated to it. Popular choices of tether contain a carbonate ester, boronic ester, silyl ether, or a silyl acetal link (silicon tethers)[4][5] which are fairly inert in many organic reactions yet can be cleaved by specific reagents. The main hurdle for this strategy to work is selecting the proper length for the tether and making sure reactive groups have an optimal orientation with respect to each other. An examples is a Pauson-Khand reaction of an alkene and an alkyne tethered together via a silyl ether [6]
In this particular reaction, the tether angle bringing the reactive groups together is effectively reduced by placing isopropyl groups on the silicon atom via the Thorpe-Ingold effect. No reaction takes place when these bulky groups are replaced by smaller methyl groups.
Another example is a photochemical [2+2]cycloaddition with two alkene groups tethered through a silicon acetal group (racemic, the other enantiomer not depicted), which is subsequently cleaved by TBAF yielding the endo-diol.
Without the tether, the exo isomer forms.[7]
See also
References
- ^ Coates, R. M.; Senter, P. D.; Baker, W. R. J. Org. Chem. 1982, 47, 3597.
- ^ Tamura, Y.; Kita, Y.; Ishibashi, H.; Ikeda, M. J. Chem. Soc. D. 1971, 19, 1167.
- ^ Corey, E. J.; Kang, M. C.; Desai, M. C.; Ghosh, A. K.; Houpis, I. N. J. Am. Chem. Soc. 1988, 110, 649.
- ^ Templated Organic Synthesis Editor(s): François Diederich, Peter J. Stang 2007 Chapter 10 Use of the Temporary Connection in Organic Synthesis (pages 274–395) Liam R. Cox and Steven V. Ley doi:10.1002/9783527613526
- ^ Bracegirdle, S.; Anderson, E. A. Chem. Soc. Rev., 2010, 39, 4114 - 4129, doi:10.1039/C0CS00007H
- ^ The use of silicon-based tethers for the Pauson-Khand reaction Dobbs A, Miller I, Martinovic S Beilstein Journal of Organic Chemistry, 2007 3:21 ( 6 July 2007 ) Article link Archived 2007-10-08 at the Wayback Machine
- ^ Diastereoselective intramolecular photochemical [2 + 2] cycloaddition reactions of tethered l-(+)-valinol derived tetrahydrophthalimides Kevin I. Booker-Milburn, Sirin Gulten and Andrew Sharpe Chem. Commun., 1997, 1385 - 1386, doi:10.1039/a702386c