Petrochemical: Difference between revisions
SimLibrarian (talk | contribs) m add navbox that links here |
|||
| (36 intermediate revisions by 26 users not shown) | |||
| Line 1: | Line 1: | ||
{{Short description|Chemical product derived from petroleum}} |
|||
[[File:TASNEE 001.jpg|thumb|upright=1.35|Petrochemical plant in |
[[File:TASNEE 001.jpg|thumb|upright=1.35|Petrochemical plant in [[Saudi Arabia]]]] |
||
'''Petrochemicals''' ( |
'''Petrochemicals''' (sometimes abbreviated as '''petchems'''<ref>Kiesche, Liz, [https://seekingalpha.com/news/3604684-royal-dutch-shell-may-take-50-stake-in-9b-indian-petchem-project-reuters "Royal Dutch Shell may take 50% stake in $9B Indian petchem project"], [[Reuters]] via ''[[Seeking Alpha]]'', August 12, 2020. Retrieved 2020-08-12.</ref>) are the [[product (chemistry)|chemical products]] obtained from [[petroleum]] by refining. Some [[chemical compound]]s made from petroleum are also obtained from other [[fossil fuel]]s, such as [[coal]] or [[natural gas]], or renewable sources such as [[maize]], [[Elaeis|palm fruit]] or [[sugar cane]]. |
||
The two most common petrochemical classes are [[alkene|olefins]] (including [[ethylene]] and [[propylene]]) and [[aromaticity|aromatics]] (including [[benzene]], [[toluene]] and [[xylene]] [[isomers]]). |
The two most common petrochemical classes are [[alkene|olefins]] (including [[ethylene]] and [[propylene]]) and [[aromaticity|aromatics]] (including [[benzene]], [[toluene]] and [[xylene]] [[isomers]]). |
||
| Line 7: | Line 8: | ||
[[Oil refinery|Oil refineries]] produce olefins and aromatics by [[fluid catalytic cracking]] of petroleum fractions. [[Chemical plant]]s produce olefins by [[steam cracking]] of [[natural gas liquids]] like [[ethane]] and [[propane]]. Aromatics are produced by [[catalytic reforming]] of [[Petroleum naphtha|naphtha]]. Olefins and aromatics are the building-blocks for a wide range of materials such as [[solvent]]s, [[detergent]]s, and [[adhesive]]s. Olefins are the basis for [[polymer]]s and [[oligomer]]s used in [[plastic]]s, [[resin]]s, [[fiber]]s, [[elastomer]]s, [[lubricant]]s, and [[gels]].<ref name="Hatch">{{cite book|author=Sami Matar and Lewis F. Hatch|title=Chemistry of Petrochemical Processes|publisher=Gulf Professional Publishing|year=2001|isbn=0-88415-315-0}}</ref><ref name="HP">{{cite journal|author=Staff |date=March 2001|title=Petrochemical Processes 2001 |journal=Hydrocarbon Processing |pages=71–246 |issn=0887-0284}}</ref> |
[[Oil refinery|Oil refineries]] produce olefins and aromatics by [[fluid catalytic cracking]] of petroleum fractions. [[Chemical plant]]s produce olefins by [[steam cracking]] of [[natural gas liquids]] like [[ethane]] and [[propane]]. Aromatics are produced by [[catalytic reforming]] of [[Petroleum naphtha|naphtha]]. Olefins and aromatics are the building-blocks for a wide range of materials such as [[solvent]]s, [[detergent]]s, and [[adhesive]]s. Olefins are the basis for [[polymer]]s and [[oligomer]]s used in [[plastic]]s, [[resin]]s, [[fiber]]s, [[elastomer]]s, [[lubricant]]s, and [[gels]].<ref name="Hatch">{{cite book|author=Sami Matar and Lewis F. Hatch|title=Chemistry of Petrochemical Processes|publisher=Gulf Professional Publishing|year=2001|isbn=0-88415-315-0}}</ref><ref name="HP">{{cite journal|author=Staff |date=March 2001|title=Petrochemical Processes 2001 |journal=Hydrocarbon Processing |pages=71–246 |issn=0887-0284}}</ref> |
||
Global ethylene production was 190 million tonnes and propylene was 120 million tonnes in 2019. |
Global ethylene production was 190 million tonnes and propylene was 120 million tonnes in 2019.<ref>{{Cite web|url=https://www.statista.com/statistics/1067372/global-ethylene-production-capacity/#:~:text=The%20production%20capacity%20of%20ubiquitous,to%20283%20million%20metric%20tons.|title = Ethylene production capacity globally 2024}}</ref> Aromatics production is approximately 70 million tonnes. The largest [[petrochemical industries]] are located in the [[United States]] and [[Western Europe]]; however, major growth in new production capacity is in the [[Middle East]] and [[Asia]]. There is substantial inter-regional petrochemical trade. |
||
Primary petrochemicals are divided into three groups depending on their [[chemical structure]]: |
Primary petrochemicals are divided into three groups depending on their [[chemical structure]]: |
||
* [[Alkene|Olefins]] includes [[Ethylene| |
* [[Alkene|Olefins]] includes [[Ethylene|ethene]], [[propene]], [[butene]]s and [[1,3-Butadiene|butadiene]]. Ethylene and propylene are important sources of [[industrial chemicals]] and [[plastics]] [[Product (business)|products]]. Butadiene is used in making [[synthetic rubber]]. |
||
* [[Aromatics]] includes [[ |
* [[Aromatics]] includes [[benzene]], [[toluene]] and [[xylene]]s, as a whole referred to as [[BTX (chemistry)|BTX]] and primarily obtained from petroleum refineries by extraction from the reformate produced in [[Catalytic reforming|catalytic reformers]] using [[Petroleum naphtha|naphtha]] obtained from petroleum refineries. Alternatively, BTX can be produced by aromatization of alkanes. Benzene is a raw material for [[dyes]] and synthetic detergents, and benzene and toluene for [[isocyanate]]s [[Methylene diphenyl diisocyanate|MDI]] and [[Toluene diisocyanate|TDI]] used in making [[polyurethanes]]. Manufacturers use [[xylene]]s to produce plastics and synthetic fibers. |
||
* [[Synthesis gas]] is a [[mixture]] of [[carbon monoxide]] and [[hydrogen]] used to |
* [[Synthesis gas]] is a [[mixture]] of [[carbon monoxide]] and [[hydrogen]] used to produce [[methanol]] and other chemicals. [[Steam cracking|Steam crackers]] are not to be confused with [[steam reforming]] plants used to produce [[hydrogen]] for [[Ammonia production|ammonia]] production. Ammonia is used to make the [[fertilizer]] [[urea]] and methanol is used as a solvent and [[chemical]] intermediate. |
||
* [[Methane]], [[ethane]], [[propane]] and [[butane]]s obtained primarily from [[Natural gas processing|natural gas processing plants]]. |
* [[Methane]], [[ethane]], [[propane]] and [[butane]]s obtained primarily from [[Natural gas processing|natural gas processing plants]]. |
||
* [[Methanol]] and [[formaldehyde]]. |
* [[Methanol]] and [[formaldehyde]]. |
||
In 2007, the amounts of ethylene and propylene produced in steam crackers were about 115 M[[Tonne|t]] (megatonnes) and 70 Mt, respectively.<ref>{{cite book|title=Proceedings of the 1st Annual Gas Processing Symposium, Volume 1: January, 2009 – Qatar| |
In 2007, the amounts of ethylene and propylene produced in steam crackers were about 115 M[[Tonne|t]] (megatonnes) and 70 Mt, respectively.<ref>{{cite book|title=Proceedings of the 1st Annual Gas Processing Symposium, Volume 1: January, 2009 – Qatar |editor1=Hassan E. Alfadala |editor2=G.V. Rex Reklaitis |editor3=Mahmoud M. El-Halwagi |publisher=Elsevier Science|year=2009|isbn=978-0-444-53292-3|edition=1st|pages=402–414}}</ref> The output ethylene capacity of large steam crackers ranged up to as much as 1.0 – 1.5 Mt per year.<ref>[http://www.technip.com/pdf/brochures/Ethylene.pdf Steam Cracking: Ethylene Production] (PDF page 3 of 12 pages)</ref> |
||
The adjacent diagram schematically depicts the major hydrocarbon sources and processes used in producing petrochemicals.<ref name="Hatch" /><ref name="HP" /><ref name="AMAP">[http://www.tsp2.org/news/Butadiene%20Supply%20AMAP%20Update.pdf SBS Polymer Supply Outlook]</ref><ref>{{cite book|title=Petroleum Refining: Refinery Operation and Management| |
The adjacent diagram schematically depicts the major hydrocarbon sources and processes used in producing petrochemicals.<ref name="Hatch" /><ref name="HP" /><ref name="AMAP">[http://www.tsp2.org/news/Butadiene%20Supply%20AMAP%20Update.pdf SBS Polymer Supply Outlook]</ref><ref>{{cite book|title=Petroleum Refining: Refinery Operation and Management|editor=Jean-Pierre Favennec |publisher=Editions Technip|year=2001|isbn=2-7108-0801-3}}</ref>[[File:Petrochem Feedstocks.png|thumb|right|upright=1.95|Petrochemical feedstock sources]] |
||
Like [[commodity chemicals]], petrochemicals are made on a very large scale. Petrochemical manufacturing units differ from commodity chemical plants in that they often produce a number of related products. Compare this with [[Specialty chemical industry|specialty chemical]] and [[fine chemical]] manufacture where products are made in discrete batch processes. |
Like [[commodity chemicals]], petrochemicals are made on a very large scale. Petrochemical manufacturing units differ from commodity chemical plants in that they often produce a number of related products. Compare this with [[Specialty chemical industry|specialty chemical]] and [[fine chemical]] manufacture where products are made in discrete batch processes. |
||
Petrochemicals are predominantly made in a few manufacturing locations around the world, for example in [[Jubail]] |
Petrochemicals are predominantly made in a few manufacturing locations around the world, for example in [[Jubail]] and [[Yanbu]] Industrial Cities in Saudi Arabia, [[Texas]] and [[Louisiana]] in the US, in [[Teesside]] in the [[Northeast of England]] in the [[United Kingdom]], in [[Tarragona]] in [[Catalonia]], in [[Rotterdam]] in the Netherlands, in [[Antwerp]] in [[Belgium]], in [[Jamnagar Refinery|Jamnagar]], [[Dahej]] in [[Gujarat]], [[India]] and in Singapore. Not all of the petrochemical or commodity chemical materials produced by the chemical industry are made in one single location but groups of related materials are often made in adjacent manufacturing plants to induce industrial symbiosis as well as material and utility efficiency and other [[economies of scale]]. This is known in [[chemical engineering]] terminology as integrated manufacturing. Specialty and fine chemical companies are sometimes found in similar manufacturing locations as petrochemicals but, in most cases, they do not need the same level of large-scale infrastructure (e.g., pipelines, storage, ports, and power, etc.) and therefore can be found in multi-sector business parks. |
||
The large |
The large-scale petrochemical manufacturing locations have clusters of manufacturing units that share utilities and large-scale infrastructures such as power stations, storage tanks, port facilities, road and rail terminals. In the United Kingdom, for example, there are four main locations for such manufacturing: near the River Mersey in North West England, on the Humber on the East coast of Yorkshire, in Grangemouth near the Firth of Forth in Scotland, and in Teesside as part of the [[Northeast of England Process Industry Cluster]] (NEPIC). To demonstrate the clustering and integration, some 50% of the United Kingdom's petrochemical and commodity chemicals are produced by the NEPIC industry cluster companies in Teesside. |
||
== History == |
== History == |
||
In 1835, [[Henri Victor Regnault]], a French chemist left [[vinyl chloride]] in the sun and found white solid at the bottom of the flask which was [[polyvinyl chloride]]. In 1839, [[Eduard Simon]] discovered polystyrene by accident by distilling [[Storax balsam|storax]]. In 1856, [[William Henry Perkin]] discovered the first synthetic dye, [[Mauveine]]. In 1888, [[Friedrich Reinitzer]], an Austrian plant scientist observed [[cholesteryl benzoate]] had two different melting points. In 1909, [[Leo Baekeland|Leo Hendrik Baekeland]] invented [[bakelite]] made from [[phenol]] and [[formaldehyde]]. In 1928, [[ |
In 1835, [[Henri Victor Regnault]], a French chemist left [[vinyl chloride]] in the sun and found white solid at the bottom of the flask which was [[polyvinyl chloride]]. In 1839, [[Eduard Simon]] discovered polystyrene by accident by distilling [[Storax balsam|storax]]. In 1856, [[William Henry Perkin]] discovered the first synthetic dye, [[Mauveine]]. In 1888, [[Friedrich Reinitzer]], an Austrian plant scientist observed [[cholesteryl benzoate]] had two different melting points. In 1909, [[Leo Baekeland|Leo Hendrik Baekeland]] invented [[bakelite]] made from [[phenol]] and [[formaldehyde]]. In 1928, [[synthetic fuel]]s were invented using [[Fischer–Tropsch process|Fischer-Tropsch process]]. In 1929, [[Walter Bock]] invented synthetic rubber [[Buna-S]] which is made up of [[styrene]] and [[1,3-Butadiene|butadiene]] and used to make car tires. In 1933, [[Otto Röhm]] polymerized the first acrylic glass [[methyl methacrylate]]. In 1935, [[Michael Perrin]] invented [[polyethylene]]. In 1937, [[Wallace Carothers|Wallace Hume Carothers]] invented [[nylon]]. In 1938, [[Otto Bayer]] invented [[polyurethane]]. In 1941, [[Roy J. Plunkett|Roy Plunkett]] invented [[Teflon]]. In 1946, he invented [[Polyester]]. [[Polyethylene terephthalate]] (PET) bottles are made from [[ethylene]] and [[P-Xylene|paraxylene]]. In 1949, Fritz Stastny turned [[polystyrene]] into foam. After World War II, [[polypropylene]] was discovered in the early 1950s. In 1965, [[Stephanie Kwolek]] invented [[Kevlar]].<ref>{{Cite web|url=https://www.petrochemistry.eu/about-petrochemistry/timeline/|title=Timeline – Petrochemicals Europe|website=www.petrochemistry.eu|language=en-US|access-date=2018-04-07}}</ref> |
||
== Olefins == |
== Olefins == |
||
The following is a partial list of |
The following is a partial list of major commercial petrochemicals and their derivatives: |
||
[[File:Petrochem1.png|thumb|upright=2.2|Chemicals produced from ethylene]] |
[[File:Petrochem1.png|thumb|upright=2.2|Chemicals produced from ethylene]] |
||
* [[ethylene]] – the simplest olefin; used as a chemical feedstock and ripening stimulant |
* [[ethylene]] – the simplest olefin; used as a chemical feedstock and ripening stimulant |
||
| Line 54: | Line 55: | ||
** [[acrylonitrile]] – useful as a monomer in forming [[Orlon]], [[Acrylonitrile butadiene styrene|ABS]] |
** [[acrylonitrile]] – useful as a monomer in forming [[Orlon]], [[Acrylonitrile butadiene styrene|ABS]] |
||
** [[polypropylene]] – [[Polymerization|polymerized]] propylene |
** [[polypropylene]] – [[Polymerization|polymerized]] propylene |
||
** [[propylene oxide]] |
** [[propylene oxide]]<ref>{{Cite journal|last1=Lee|first1=Eo Jin|last2=Lee|first2=Jong Won|last3=Lee|first3=Joongwon|last4=Min|first4=Hyung-Ki|last5=Yi|first5=Jongheop|last6=Song|first6=In Kyu|last7=Kim|first7=Do Heui|date=2018-06-01|title=Ag-(Mo-W)/ZrO2 catalysts for the production of propylene oxide: Effect of pH in the preparation of ZrO2 support|url=http://www.sciencedirect.com/science/article/pii/S1566736718301353|journal=Catalysis Communications|language=en|volume=111|pages=80–83|doi=10.1016/j.catcom.2018.04.005|s2cid=103189174|issn=1566-7367}}</ref> |
||
***polyether polyol – used in the production of polyurethanes |
***polyether polyol – used in the production of polyurethanes |
||
***[[propylene glycol]] – used in engine coolant <ref>{{Cite patent|title=Anti-freeze solution for internal combustion engines| |
***[[propylene glycol]] – used in engine coolant <ref>{{Cite patent|country=HU|number=209546B|title=Anti-freeze solution for internal combustion engines|status=patent|pubdate=1994-07-28|fdate=1990-11-12|invent1=Forstner|invent2=Gal|invent3=Feher|invent4=Berkes|inventor1-first=Janos|inventor2-first=Lajos|inventor3-first=Pal|inventor4-first=Tiborne}}</ref> and aircraft deicer fluid |
||
***[[glycol ether]]s – from the condensation of glycols |
***[[glycol ether]]s – from the condensation of glycols |
||
** [[acrylic acid]] |
|||
** [[acrylic acid]] <ref>{{Cite journal|year=2012|title=Surface chemistry of phase-pure M1 MoVTeNb oxide during operation in selective oxidation of propane to acrylic acid|url=https://pure.mpg.de/rest/items/item_1108560_8/component/file_1402724/content|journal=J. Catal.|volume=285|pages=48–60|doi=10.1016/j.jcat.2011.09.012|hdl=11858/00-001M-0000-0012-1BEB-F|last1=Hävecker|first1=Michael|last2=Wrabetz|first2=Sabine|last3=Kröhnert|first3=Jutta|last4=Csepei|first4=Lenard-Istvan|last5=Naumann d'Alnoncourt|first5=Raoul|last6=Kolen'Ko|first6=Yury V.|last7=Girgsdies|first7=Frank|last8=Schlögl|first8=Robert|last9=Trunschke|first9=Annette|hdl-access=free}}</ref><ref>{{Cite journal|year=2014|title=The reaction network in propane oxidation over phase-pure MoVTeNb M1 oxide catalysts.|url=https://pure.mpg.de/rest/items/item_1896844_6/component/file_1896843/content|journal=J. Catal.|volume=311|pages=369–385|doi=10.1016/j.jcat.2013.12.008|hdl=11858/00-001M-0000-0014-F434-5|last1=Naumann d'Alnoncourt|first1=Raoul|last2=Csepei|first2=Lénárd-István|last3=Hävecker|first3=Michael|last4=Girgsdies|first4=Frank|last5=Schuster|first5=Manfred E.|last6=Schlögl|first6=Robert|last7=Trunschke|first7=Annette|hdl-access=free}}</ref><ref>{{Cite book|url=https://pure.mpg.de/rest/items/item_1199619_5/component/file_1199618/content|title=Kinetic studies of propane oxidation on Mo and V based mixed oxide catalysts|year=2011}}</ref> |
|||
***[[acrylic polymer]]s |
***[[acrylic polymer]]s |
||
** [[allyl chloride]] |
** [[allyl chloride]] |
||
| Line 117: | Line 118: | ||
*** [[isophthalic acid]] |
*** [[isophthalic acid]] |
||
**** [[Alkyd|alkyd resins]] |
**** [[Alkyd|alkyd resins]] |
||
**** [[Polyamide-imide| |
**** [[Polyamide-imide|polyamide resins]] |
||
**** [[Polyester resin| |
**** [[Polyester resin|unsaturated polyesters]] |
||
==List of petrochemicals== |
==List of petrochemicals== |
||
| Line 145: | Line 146: | ||
*[[Instrumentation in petrochemical industries]] |
*[[Instrumentation in petrochemical industries]] |
||
*[[Organization of the Petroleum Exporting Countries]] |
*[[Organization of the Petroleum Exporting Countries]] |
||
*[[Asia Petrochemical Industry Conference]](APIC) |
*[[Asia Petrochemical Industry Conference]] (APIC) |
||
*[[Northeast of England Process Industry Cluster]](NEPIC) |
*[[Northeast of England Process Industry Cluster]] (NEPIC) |
||
==References== |
==References== |
||
{{ |
{{Reflist|30em}} |
||
==External links== |
==External links== |
||
{{ |
{{Commons category|Petrochemicals}}{{Branches of chemistry}}{{authority control}} |
||
[[Category:Petrochemicals| ]] |
[[Category:Petrochemicals| ]] |
||
__FORCETOC__ |
|||
Latest revision as of 02:38, 11 February 2024

Petrochemicals (sometimes abbreviated as petchems[1]) are the chemical products obtained from petroleum by refining. Some chemical compounds made from petroleum are also obtained from other fossil fuels, such as coal or natural gas, or renewable sources such as maize, palm fruit or sugar cane.
The two most common petrochemical classes are olefins (including ethylene and propylene) and aromatics (including benzene, toluene and xylene isomers).
Oil refineries produce olefins and aromatics by fluid catalytic cracking of petroleum fractions. Chemical plants produce olefins by steam cracking of natural gas liquids like ethane and propane. Aromatics are produced by catalytic reforming of naphtha. Olefins and aromatics are the building-blocks for a wide range of materials such as solvents, detergents, and adhesives. Olefins are the basis for polymers and oligomers used in plastics, resins, fibers, elastomers, lubricants, and gels.[2][3]
Global ethylene production was 190 million tonnes and propylene was 120 million tonnes in 2019.[4] Aromatics production is approximately 70 million tonnes. The largest petrochemical industries are located in the United States and Western Europe; however, major growth in new production capacity is in the Middle East and Asia. There is substantial inter-regional petrochemical trade.
Primary petrochemicals are divided into three groups depending on their chemical structure:
- Olefins includes ethene, propene, butenes and butadiene. Ethylene and propylene are important sources of industrial chemicals and plastics products. Butadiene is used in making synthetic rubber.
- Aromatics includes benzene, toluene and xylenes, as a whole referred to as BTX and primarily obtained from petroleum refineries by extraction from the reformate produced in catalytic reformers using naphtha obtained from petroleum refineries. Alternatively, BTX can be produced by aromatization of alkanes. Benzene is a raw material for dyes and synthetic detergents, and benzene and toluene for isocyanates MDI and TDI used in making polyurethanes. Manufacturers use xylenes to produce plastics and synthetic fibers.
- Synthesis gas is a mixture of carbon monoxide and hydrogen used to produce methanol and other chemicals. Steam crackers are not to be confused with steam reforming plants used to produce hydrogen for ammonia production. Ammonia is used to make the fertilizer urea and methanol is used as a solvent and chemical intermediate.
- Methane, ethane, propane and butanes obtained primarily from natural gas processing plants.
- Methanol and formaldehyde.
In 2007, the amounts of ethylene and propylene produced in steam crackers were about 115 Mt (megatonnes) and 70 Mt, respectively.[5] The output ethylene capacity of large steam crackers ranged up to as much as 1.0 – 1.5 Mt per year.[6]
The adjacent diagram schematically depicts the major hydrocarbon sources and processes used in producing petrochemicals.[2][3][7][8]
Like commodity chemicals, petrochemicals are made on a very large scale. Petrochemical manufacturing units differ from commodity chemical plants in that they often produce a number of related products. Compare this with specialty chemical and fine chemical manufacture where products are made in discrete batch processes.
Petrochemicals are predominantly made in a few manufacturing locations around the world, for example in Jubail and Yanbu Industrial Cities in Saudi Arabia, Texas and Louisiana in the US, in Teesside in the Northeast of England in the United Kingdom, in Tarragona in Catalonia, in Rotterdam in the Netherlands, in Antwerp in Belgium, in Jamnagar, Dahej in Gujarat, India and in Singapore. Not all of the petrochemical or commodity chemical materials produced by the chemical industry are made in one single location but groups of related materials are often made in adjacent manufacturing plants to induce industrial symbiosis as well as material and utility efficiency and other economies of scale. This is known in chemical engineering terminology as integrated manufacturing. Specialty and fine chemical companies are sometimes found in similar manufacturing locations as petrochemicals but, in most cases, they do not need the same level of large-scale infrastructure (e.g., pipelines, storage, ports, and power, etc.) and therefore can be found in multi-sector business parks.
The large-scale petrochemical manufacturing locations have clusters of manufacturing units that share utilities and large-scale infrastructures such as power stations, storage tanks, port facilities, road and rail terminals. In the United Kingdom, for example, there are four main locations for such manufacturing: near the River Mersey in North West England, on the Humber on the East coast of Yorkshire, in Grangemouth near the Firth of Forth in Scotland, and in Teesside as part of the Northeast of England Process Industry Cluster (NEPIC). To demonstrate the clustering and integration, some 50% of the United Kingdom's petrochemical and commodity chemicals are produced by the NEPIC industry cluster companies in Teesside.
History[edit]
In 1835, Henri Victor Regnault, a French chemist left vinyl chloride in the sun and found white solid at the bottom of the flask which was polyvinyl chloride. In 1839, Eduard Simon discovered polystyrene by accident by distilling storax. In 1856, William Henry Perkin discovered the first synthetic dye, Mauveine. In 1888, Friedrich Reinitzer, an Austrian plant scientist observed cholesteryl benzoate had two different melting points. In 1909, Leo Hendrik Baekeland invented bakelite made from phenol and formaldehyde. In 1928, synthetic fuels were invented using Fischer-Tropsch process. In 1929, Walter Bock invented synthetic rubber Buna-S which is made up of styrene and butadiene and used to make car tires. In 1933, Otto Röhm polymerized the first acrylic glass methyl methacrylate. In 1935, Michael Perrin invented polyethylene. In 1937, Wallace Hume Carothers invented nylon. In 1938, Otto Bayer invented polyurethane. In 1941, Roy Plunkett invented Teflon. In 1946, he invented Polyester. Polyethylene terephthalate (PET) bottles are made from ethylene and paraxylene. In 1949, Fritz Stastny turned polystyrene into foam. After World War II, polypropylene was discovered in the early 1950s. In 1965, Stephanie Kwolek invented Kevlar.[9]
Olefins[edit]
The following is a partial list of major commercial petrochemicals and their derivatives:

- ethylene – the simplest olefin; used as a chemical feedstock and ripening stimulant
- polyethylene – polymerized ethylene; LDPE, HDPE, LLDPE
- ethanol – via ethylene hydration (chemical reaction adding water) of ethylene
- ethylene oxide – via ethylene oxidation
- ethylene glycol – via ethylene oxide hydration
- engine coolant – ethylene glycol, water and inhibitor mixture
- polyesters – any of several polymers with ester linkages in the main chain
- glycol ethers – via glycol condescension
- ethoxylates
- ethylene glycol – via ethylene oxide hydration
- vinyl acetate[10]
- 1,2-dichloroethane
- trichloroethylene
- tetrachloroethylene – also called perchloroethylene; used as a dry cleaning solvent and degreaser
- vinyl chloride – monomer for polyvinyl chloride
- polyvinyl chloride (PVC) – a type of plastic used for piping, tubing, other things
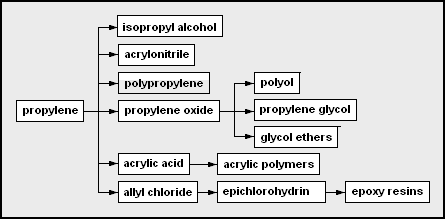
- propylene – used as a monomer and a chemical feedstock
- isopropyl alcohol – 2-propanol; often used as a solvent or rubbing alcohol
- acrylonitrile – useful as a monomer in forming Orlon, ABS
- polypropylene – polymerized propylene
- propylene oxide[11]
- polyether polyol – used in the production of polyurethanes
- propylene glycol – used in engine coolant [12] and aircraft deicer fluid
- glycol ethers – from the condensation of glycols
- acrylic acid
- allyl chloride
- epichlorohydrin – chloro-oxirane; used in epoxy resin formation
- epoxy resins – a type of polymerizing glue from bisphenol A, epichlorohydrin, and some amine
- epichlorohydrin – chloro-oxirane; used in epoxy resin formation
- butene
- isomers of butylene – useful as monomers or co-monomers
- isobutylene – feed for making methyl tert-butyl ether (MTBE) or monomer for copolymerization with a low percentage of isoprene to make butyl rubber
- 1,3-butadiene (or buta-1,3-diene) – a diene often used as a monomer or co-monomer for polymerization to elastomers such as polybutadiene, styrene-butadiene rubber, or a plastic such as acrylonitrile-butadiene-styrene (ABS)
- synthetic rubbers – synthetic elastomers made of any one or more of several petrochemical (usually) monomers such as 1,3-butadiene, styrene, isobutylene, isoprene, chloroprene; elastomeric polymers are often made with a high percentage of conjugated diene monomers such as 1,3-butadiene, isoprene, or chloroprene
- isomers of butylene – useful as monomers or co-monomers
- higher olefins
- polyolefins – such poly-alpha-olefins, which are used as lubricants
- alpha-olefins – used as monomers, co-monomers, and other chemical precursors. For example, a small amount of 1-hexene can be copolymerized with ethylene into a more flexible form of polyethylene.
- other higher olefins
- detergent alcohols
Aromatics[edit]

- benzene – the simplest aromatic hydrocarbon
- ethylbenzene – made from benzene and ethylene
- styrene – made by dehydrogenation of ethylbenzene; used as a monomer
- polystyrenes – polymers with styrene as a monomer
- styrene – made by dehydrogenation of ethylbenzene; used as a monomer
- cumene – isopropylbenzene; a feedstock in the cumene process
- phenol – hydroxybenzene; often made by the cumene process
- acetone – dimethyl ketone; also often made by the cumene process
- bisphenol A – a type of "double" phenol used in polymerization in epoxy resins and making a common type of polycarbonate
- epoxy resins – a type of polymerizing glue from bisphenol A, epichlorohydrin, and some amine
- polycarbonate – a plastic polymer made from bisphenol A and phosgene (carbonyl dichloride)
- solvents – liquids used for dissolving materials; examples often made from petrochemicals include ethanol, isopropyl alcohol, acetone, benzene, toluene, xylenes
- cyclohexane – a 6-carbon aliphatic cyclic hydrocarbon sometimes used as a non-polar solvent
- adipic acid – a 6-carbon dicarboxylic acid, which can be a precursor used as a co-monomer together with a diamine to form an alternating copolymer form of nylon.
- nylons – types of polyamides, some are alternating copolymers formed from copolymerizing dicarboxylic acid or derivatives with diamines
- caprolactam – a 6-carbon cyclic amide
- nylons – types of polyamides, some are from polymerizing caprolactam
- adipic acid – a 6-carbon dicarboxylic acid, which can be a precursor used as a co-monomer together with a diamine to form an alternating copolymer form of nylon.
- nitrobenzene – can be made by single nitration of benzene
- aniline – aminobenzene
- methylene diphenyl diisocyanate (MDI) – used as a co-monomer with diols or polyols to form polyurethanes or with di- or polyamines to form polyureas
- aniline – aminobenzene
- alkylbenzene – a general type of aromatic hydrocarbon, which can be used as a precursor for a sulfonate surfactant (detergent)
- detergents – often include surfactants types such as alkylbenzene sulfonates and nonylphenol ethoxylates
- chlorobenzene
- ethylbenzene – made from benzene and ethylene
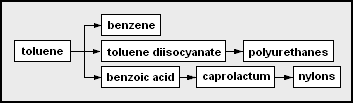
- toluene – methylbenzene; can be a solvent or precursor for other chemicals
- benzene
- toluene diisocyanate (TDI) – used as co-monomers with polyether polyols to form polyurethanes or with di- or polyamines to form polyureas polyurethanes
- benzoic acid – carboxybenzene
- mixed xylenes – any of three dimethylbenzene isomers, could be a solvent but more often precursor chemicals
- ortho-xylene – both methyl groups can be oxidized to form (ortho-)phthalic acid
- para-xylene – both methyl groups can be oxidized to form terephthalic acid
- dimethyl terephthalate – can be copolymerized to form certain polyesters
- polyesters – although there can be many types, polyethylene terephthalate is made from petrochemical products and is very widely used in petrol stations
- purified terephthalic acid – often copolymerized to form polyethylene terephthalate
- dimethyl terephthalate – can be copolymerized to form certain polyesters
- meta-xylene
List of petrochemicals[edit]
See also[edit]
- Petroleum
- Petroleum products
- Instrumentation in petrochemical industries
- Organization of the Petroleum Exporting Countries
- Asia Petrochemical Industry Conference (APIC)
- Northeast of England Process Industry Cluster (NEPIC)
References[edit]
- ^ Kiesche, Liz, "Royal Dutch Shell may take 50% stake in $9B Indian petchem project", Reuters via Seeking Alpha, August 12, 2020. Retrieved 2020-08-12.
- ^ a b Sami Matar and Lewis F. Hatch (2001). Chemistry of Petrochemical Processes. Gulf Professional Publishing. ISBN 0-88415-315-0.
- ^ a b Staff (March 2001). "Petrochemical Processes 2001". Hydrocarbon Processing: 71–246. ISSN 0887-0284.
- ^ "Ethylene production capacity globally 2024".
- ^ Hassan E. Alfadala; G.V. Rex Reklaitis; Mahmoud M. El-Halwagi, eds. (2009). Proceedings of the 1st Annual Gas Processing Symposium, Volume 1: January, 2009 – Qatar (1st ed.). Elsevier Science. pp. 402–414. ISBN 978-0-444-53292-3.
- ^ Steam Cracking: Ethylene Production (PDF page 3 of 12 pages)
- ^ SBS Polymer Supply Outlook
- ^ Jean-Pierre Favennec, ed. (2001). Petroleum Refining: Refinery Operation and Management. Editions Technip. ISBN 2-7108-0801-3.
- ^ "Timeline – Petrochemicals Europe". www.petrochemistry.eu. Retrieved 2018-04-07.
- ^ Han, Y. -F.; Wang, J. -H.; Kumar, D.; Yan, Z.; Goodman, D. W. (2005-06-10). "A kinetic study of vinyl acetate synthesis over Pd-based catalysts: kinetics of vinyl acetate synthesis over Pd–Au/SiO2 and Pd/SiO2 catalysts". Journal of Catalysis. 232 (2): 467–475. doi:10.1016/j.jcat.2005.04.001. ISSN 0021-9517.
- ^ Lee, Eo Jin; Lee, Jong Won; Lee, Joongwon; Min, Hyung-Ki; Yi, Jongheop; Song, In Kyu; Kim, Do Heui (2018-06-01). "Ag-(Mo-W)/ZrO2 catalysts for the production of propylene oxide: Effect of pH in the preparation of ZrO2 support". Catalysis Communications. 111: 80–83. doi:10.1016/j.catcom.2018.04.005. ISSN 1566-7367. S2CID 103189174.
- ^ HU patent 209546B, Forstner, Janos; Gal, Lajos & Feher, Pal et al., "Anti-freeze solution for internal combustion engines", published 1994-07-28